Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment
- PMID: 24600000
- PMCID: PMC4093845
- DOI: 10.1128/JVI.00278-14
Herpes simplex virus 1 protein UL37 interacts with viral glycoprotein gK and membrane protein UL20 and functions in cytoplasmic virion envelopment
Abstract
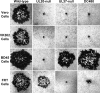
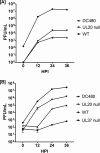
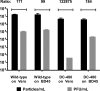
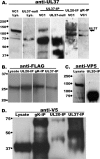
We have shown that glycoprotein K (gK) and its interacting partner, the UL20 protein, play crucial roles in virion envelopment. Specifically, virions lacking either gK or UL20 fail to acquire an envelope, thus causing accumulation of capsids in the cytoplasm of infected cells. The herpes simplex virus 1 (HSV-1) UL37 protein has also been implicated in cytoplasmic virion envelopment. To further investigate the role of UL37 in virion envelopment, the recombinant virus DC480 was constructed by insertion of a 12-amino-acid protein C (protC) epitope tag within the UL37 amino acid sequence immediately after amino acid 480. The DC480 mutant virus expressed full-size UL37 as detected by the anti-protC antibody in Western immunoblots, accumulated unenveloped capsids in the cytoplasm of infected cells, and produced very small plaques on African green monkey kidney (Vero) cells that were similar in size to those produced by the UL20-null and UL37-null viruses. The DC480 virus replicated nearly 4 log less efficiently than the parental wild-type virus when grown on Vero cells. However, DC480 mutant virus titers increased nearly 20-fold when the virus was grown on FRT cells engineered to express the UL20 gene in comparison to the titers on Vero cells, while the UL37-null virus replicated approximately 20-fold less efficiently than the DC480 virus on FRT cells. Coimmunoprecipitation experiments and proximity ligation assays showed that gK and UL20 interact with the UL37 protein in infected cells. Collectively, these results indicate that UL37 interacts with the gK-UL20 protein complex to facilitate cytoplasmic virion envelopment.
Importance: Herpes simplex viruses acquire their final envelopes by budding into cytoplasmic membranes derived from the trans-Golgi network (TGN). The tegument proteins UL36 and UL37 are known to be transported to the TGN sites of virus envelopment and to function in virion envelopment, since mutants lacking UL37 accumulate capsids in the cytoplasm that are unable to bud into TGN membranes. Viral glycoprotein K (gK) also functions in cytoplasmic envelopment, in a protein complex with the membrane-associated protein UL20 (UL20mp). This work shows for the first time that the UL37 protein functionally interacts with gK and UL20 to facilitate cytoplasmic virion envelopment. This work may lead to the design of specific drugs that can interrupt UL37 interactions with the gK-UL20 protein complex, providing new ways to combat herpesviral infections.
Figures


Similar articles
-
Phenylalanine residues at the carboxyl terminus of the herpes simplex virus 1 UL20 membrane protein regulate cytoplasmic virion envelopment and infectious virus production.J Virol. 2014 Jul;88(13):7618-27. doi: 10.1128/JVI.00657-14. Epub 2014 Apr 23. J Virol. 2014. PMID: 24760889 Free PMC article.
-
UL20 protein functions precede and are required for the UL11 functions of herpes simplex virus type 1 cytoplasmic virion envelopment.J Virol. 2007 Apr;81(7):3097-108. doi: 10.1128/JVI.02201-06. Epub 2007 Jan 10. J Virol. 2007. PMID: 17215291 Free PMC article.
-
Herpes Simplex Virus 1 UL37 Protein Tyrosine Residues Conserved among All Alphaherpesviruses Are Required for Interactions with Glycoprotein K, Cytoplasmic Virion Envelopment, and Infectious Virus Production.J Virol. 2016 Oct 28;90(22):10351-10361. doi: 10.1128/JVI.01202-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27630233 Free PMC article.
-
Herpesvirus Nuclear Egress across the Outer Nuclear Membrane.Viruses. 2021 Nov 24;13(12):2356. doi: 10.3390/v13122356. Viruses. 2021. PMID: 34960625 Free PMC article. Review.
-
Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion.Viruses. 2021 Sep 16;13(9):1849. doi: 10.3390/v13091849. Viruses. 2021. PMID: 34578430 Free PMC article. Review.
Cited by
-
Human Cytomegalovirus pUL47 Modulates Tegumentation and Capsid Accumulation at the Viral Assembly Complex.J Virol. 2015 Jul;89(14):7314-28. doi: 10.1128/JVI.00603-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948747 Free PMC article.
-
Advances in the immunoescape mechanisms exploited by alphaherpesviruses.Front Microbiol. 2024 Jun 19;15:1392814. doi: 10.3389/fmicb.2024.1392814. eCollection 2024. Front Microbiol. 2024. PMID: 38962133 Free PMC article. Review.
-
Role of Herpes Simplex Virus Type 1 (HSV-1) Glycoprotein K (gK) Pathogenic CD8+ T Cells in Exacerbation of Eye Disease.Front Immunol. 2018 Dec 7;9:2895. doi: 10.3389/fimmu.2018.02895. eCollection 2018. Front Immunol. 2018. PMID: 30581441 Free PMC article. Review.
-
Assembly and Egress of an Alphaherpesvirus Clockwork.Adv Anat Embryol Cell Biol. 2017;223:171-193. doi: 10.1007/978-3-319-53168-7_8. Adv Anat Embryol Cell Biol. 2017. PMID: 28528444 Free PMC article. Review.
-
Insights into herpesvirus tegument organization from structural analyses of the 970 central residues of HSV-1 UL36 protein.J Biol Chem. 2015 Apr 3;290(14):8820-33. doi: 10.1074/jbc.M114.612838. Epub 2015 Feb 12. J Biol Chem. 2015. PMID: 25678705 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

