TWEAK promotes peritoneal inflammation
- PMID: 24599047
- PMCID: PMC3944020
- DOI: 10.1371/journal.pone.0090399
TWEAK promotes peritoneal inflammation
Abstract
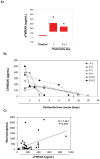
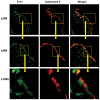
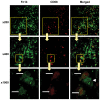
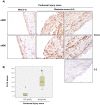
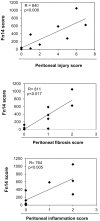
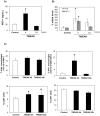
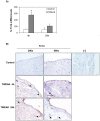
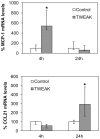
Peritoneal dialysis (PD) is complicated by peritonitis episodes that cause loss of mesothelium and eventually sclerosing peritonitis. An improved understanding of the molecular contributors to peritoneal injury and defense may increase the therapeutic armamentarium to optimize peritoneal defenses while minimizing peritoneal injury. There is no information on the expression and function of the cytokine TWEAK and its receptor Fn14 during peritoneal injury. Fn14 expression and soluble TWEAK levels were measured in human PD peritoneal effluent cells or fluids with or without peritonitis. Fn14 expression was also analyzed in peritoneal biopsies from PD patients. Actions of intraperitoneal TWEAK were studied in mice in vivo. sTWEAK levels were increased in peritoneal effluent in PD peritonitis. Effluent sTWEAK levels correlated with the number of peritoneal macrophages (r=0.491, p=0.002). Potential TWEAK targets that express the receptor Fn14 include mesothelial cells and macrophages, as demonstrated by flow cytometry of peritoneal effluents and by analysis of peritoneal biopsies. Peritoneal biopsy Fn14 correlated with mesothelial injury, fibrosis and inflammation, suggesting a potential deleterious effect of TWEAK/Fn14. In this regard, intraperitoneal TWEAK administration to mice promoted peritoneal inflammation characterized by increased peritoneal effluent MCP-1, Fn14 and Gr1+ macrophages, increased mesothelial Fn14, MCP-1 and CCL21 expression and submesothelial tissue macrophage recruitment. Taken together these data suggest that the TWEAK/Fn14 system may promote inflammation and tissue injury during peritonitis and PD.
Conflict of interest statement
Figures

Similar articles
-
Fn14 in podocytes and proteinuric kidney disease.Biochim Biophys Acta. 2013 Dec;1832(12):2232-43. doi: 10.1016/j.bbadis.2013.08.010. Epub 2013 Aug 30. Biochim Biophys Acta. 2013. PMID: 23999007
-
Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells.J Immunol. 2006 Feb 1;176(3):1889-98. doi: 10.4049/jimmunol.176.3.1889. J Immunol. 2006. PMID: 16424220
-
TWEAK and Fn14 expression in the pathogenesis of joint inflammation and bone erosion in rheumatoid arthritis.Arthritis Res Ther. 2011 Mar 24;13(2):R51. doi: 10.1186/ar3294. Arthritis Res Ther. 2011. PMID: 21435232 Free PMC article.
-
TWEAK, a multifunctional cytokine in kidney injury.Kidney Int. 2011 Oct;80(7):708-18. doi: 10.1038/ki.2011.180. Epub 2011 Jun 22. Kidney Int. 2011. PMID: 21697814 Review.
-
TWEAKing renal injury.Front Biosci. 2008 Jan 1;13:580-9. doi: 10.2741/2703. Front Biosci. 2008. PMID: 17981571 Review.
Cited by
-
IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis.Biomolecules. 2020 Sep 24;10(10):1361. doi: 10.3390/biom10101361. Biomolecules. 2020. PMID: 32987705 Free PMC article. Review.
-
TWEAK activation of the non-canonical NF-κB signaling pathway differentially regulates melanoma and prostate cancer cell invasion.Oncotarget. 2016 Dec 6;7(49):81474-81492. doi: 10.18632/oncotarget.13034. Oncotarget. 2016. PMID: 27821799 Free PMC article.
-
Fn14 deficiency protects lupus-prone mice from histological lupus erythematosus-like skin inflammation induced by ultraviolet light.Exp Dermatol. 2016 Dec;25(12):969-976. doi: 10.1111/exd.13108. Exp Dermatol. 2016. PMID: 27305603 Free PMC article.
-
Bcl3: a regulator of NF-κB inducible by TWEAK in acute kidney injury with anti-inflammatory and antiapoptotic properties in tubular cells.Exp Mol Med. 2017 Jul 7;49(7):e352. doi: 10.1038/emm.2017.89. Exp Mol Med. 2017. PMID: 28684863 Free PMC article.
-
Administration of Intravenous Inf liximab for Prevention of Peritoneal Adhesions Formation in Rats.Bull Emerg Trauma. 2015 Jul;3(3):97-103. Bull Emerg Trauma. 2015. PMID: 27162911 Free PMC article.
References
-
- Santamaría B, Sanz A, Justo P, Catalán M, Sánchez-Niño MD, et al. (2006) Peritoneal defence—lessons learned which apply to diabetes complications. Nephrol Dial Transplant 21 Suppl 2 ii12–15. - PubMed
-
- Flessner MF (2007) Inflammation from sterile dialysis solutions and the longevity of the peritoneal barrier. Clin Nephrol 68: 341–348. - PubMed
-
- Lai KN, Leung JC (2010) Inflammation in peritoneal dialysis. Nephron Clin Pract 116: c11–18. - PubMed
-
- Blake PG (2010) Peritonitis and catheter guidelines—a 2010 update. Perit Dial Int 30: 391–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

