Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task
- PMID: 24598074
- PMCID: PMC4114928
- DOI: 10.4161/sgtp.27952
Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task
Abstract
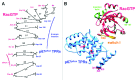
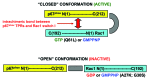
The superoxide-generating NADPH oxidase of phagocytes consists of the membrane-associated cytochrome b 558 (a heterodimer of Nox2 and p22(phox)) and 4 cytosolic components: p47(phox), p67(phox), p40(phox), and the small GTPase, Rac, in complex with RhoGDI. Superoxide is produced by the NADPH-driven reduction of molecular oxygen, via a redox gradient located in Nox2. Electron flow in Nox2 is initiated by interaction with cytosolic components, which translocate to the membrane, p67(phox) playing the central role. The participation of Rac is expressed in the following sequence: (1) Translocation of the RacGDP-RhoGDI complex to the membrane; (2) Dissociation of RacGDP from RhoGDI; (3) GDP to GTP exchange on Rac, mediated by a guanine nucleotide exchange factor; (4) Binding of RacGTP to p67(phox); (5) Induction of a conformational change in p67(phox), promoting interaction with Nox2. The particular involvement of Rac in NADPH oxidase assembly serves as a paradigm for signaling by Rho GTPases, in general.
Keywords: GEF; NADPH oxidase; Rac1; Rac2; Rho GTPases; RhoGDI; cell-free system; cytochrome b558; p67phox; superoxide.
Figures

Similar articles
-
Arachidonic acid induces direct interaction of the p67(phox)-Rac complex with the phagocyte oxidase Nox2, leading to superoxide production.J Biol Chem. 2014 Sep 5;289(36):24874-84. doi: 10.1074/jbc.M114.581785. Epub 2014 Jul 23. J Biol Chem. 2014. PMID: 25056956 Free PMC article.
-
Liposomes comprising anionic but not neutral phospholipids cause dissociation of Rac(1 or 2) x RhoGDI complexes and support amphiphile-independent NADPH oxidase activation by such complexes.J Biol Chem. 2006 Jul 14;281(28):19204-19. doi: 10.1074/jbc.M600042200. Epub 2006 May 15. J Biol Chem. 2006. PMID: 16702219
-
Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly.J Biol Chem. 2000 Dec 22;275(51):40073-81. doi: 10.1074/jbc.M006013200. J Biol Chem. 2000. PMID: 11007780
-
Cell-Free NADPH Oxidase Activation Assays: A Triumph of Reductionism.Methods Mol Biol. 2020;2087:325-411. doi: 10.1007/978-1-0716-0154-9_23. Methods Mol Biol. 2020. PMID: 31729001 Review.
-
Role of the small GTPase Rac in p22phox-dependent NADPH oxidases.Biochimie. 2007 Sep;89(9):1133-44. doi: 10.1016/j.biochi.2007.05.003. Epub 2007 May 17. Biochimie. 2007. PMID: 17583407 Review.
Cited by
-
Rho GTPases at the crossroad of signaling networks in mammals.Small GTPases. 2015;6(2):43-8. doi: 10.1080/21541248.2015.1044811. Epub 2015 Jun 25. Small GTPases. 2015. PMID: 26110743 Free PMC article. No abstract available.
-
Mechanistic computational modeling of the kinetics and regulation of NADPH oxidase 2 assembly and activation facilitating superoxide production.Free Radic Res. 2020 Oct;54(10):695-721. doi: 10.1080/10715762.2020.1836368. Epub 2020 Nov 9. Free Radic Res. 2020. PMID: 33059489 Free PMC article.
-
NOX4 Mediates Pseudomonas aeruginosa-Induced Nuclear Reactive Oxygen Species Generation and Chromatin Remodeling in Lung Epithelium.Antioxidants (Basel). 2021 Mar 17;10(3):477. doi: 10.3390/antiox10030477. Antioxidants (Basel). 2021. PMID: 33802941 Free PMC article.
-
Nicotinamide adenine dinucleotide phosphate oxidase in pancreatic diseases: Mechanisms and future perspectives.World J Gastroenterol. 2024 Feb 7;30(5):429-439. doi: 10.3748/wjg.v30.i5.429. World J Gastroenterol. 2024. PMID: 38414585 Free PMC article. Review.
-
Rho-Family Small GTPases: From Highly Polarized Sensory Neurons to Cancer Cells.Cells. 2019 Jan 28;8(2):92. doi: 10.3390/cells8020092. Cells. 2019. PMID: 30696065 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
