Global Inhibition of DC Priming Capacity in the Spleen of Self-Antigen Vaccinated Mice Requires IL-10
- PMID: 24596571
- PMCID: PMC3925839
- DOI: 10.3389/fimmu.2014.00059
Global Inhibition of DC Priming Capacity in the Spleen of Self-Antigen Vaccinated Mice Requires IL-10
Abstract
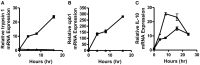
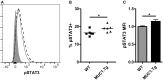
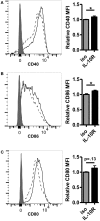
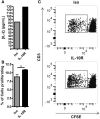
Dendritic cells (DC) in the spleen are highly activated following intravenous vaccination with a foreign-antigen, promoting expansion of effector T cells, but remain phenotypically and functionally immature after vaccination with a self-antigen. Up-regulation or suppression of expression of a cohort of pancreatic enzymes 24-72 h post-vaccination can be used as a biomarker of stimulatory versus tolerogenic DC, respectively. Here we show, using MUC1 transgenic mice and a vaccine based on the MUC1 peptide, which these mice perceive as a self-antigen, that the difference in enzyme expression that predicts whether DC will promote immune response or immune tolerance is seen as early as 4-8 h following vaccination. We also identify early production of IL-10 as a predominant factor that both correlates with this early-time point and controls DC function. Pre-treating mice with an antibody against the IL-10 receptor prior to vaccination results in DC that up-regulate CD40, CD80, and CD86 and promote stronger IFNγ+ T cell responses. This study suggests that transient inhibition of IL-10 prior to vaccination could improve responses to cancer vaccines that utilize self-tumor antigens.
Keywords: IL-10; MUC1; T cell response; cancer vaccine; dendritic cells.
Figures
Similar articles
-
Antigen choice determines vaccine-induced generation of immunogenic versus tolerogenic dendritic cells that are marked by differential expression of pancreatic enzymes.J Immunol. 2013 Apr 1;190(7):3319-27. doi: 10.4049/jimmunol.1203321. Epub 2013 Feb 18. J Immunol. 2013. PMID: 23420890 Free PMC article.
-
[Effect of neonatal BCG vaccination on phenotype and function of splenic dendritic cells of BALB/c mice].Zhonghua Er Ke Za Zhi. 2008 Oct;46(10):784-8. Zhonghua Er Ke Za Zhi. 2008. PMID: 19099886 Chinese.
-
Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb.Transplantation. 1999 Sep 27;68(6):747-57. doi: 10.1097/00007890-199909270-00006. Transplantation. 1999. PMID: 10515374 Free PMC article.
-
Novel regulation of CD80/CD86-induced phosphatidylinositol 3-kinase signaling by NOTCH1 protein in interleukin-6 and indoleamine 2,3-dioxygenase production by dendritic cells.J Biol Chem. 2014 Mar 14;289(11):7747-62. doi: 10.1074/jbc.M113.519686. Epub 2014 Jan 10. J Biol Chem. 2014. PMID: 24415757 Free PMC article.
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
Cited by
-
Dendritic cell control of immune responses.Front Immunol. 2015 Feb 5;6:42. doi: 10.3389/fimmu.2015.00042. eCollection 2015. Front Immunol. 2015. PMID: 25699058 Free PMC article. No abstract available.
-
Insights Into Dendritic Cells in Cancer Immunotherapy: From Bench to Clinical Applications.Front Cell Dev Biol. 2021 Jun 28;9:686544. doi: 10.3389/fcell.2021.686544. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34262904 Free PMC article. Review.
References
-
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol (1997) 159:4772–80 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

