Bone morphogenetic protein signaling protects against cerulein-induced pancreatic fibrosis
- PMID: 24586530
- PMCID: PMC3931685
- DOI: 10.1371/journal.pone.0089114
Bone morphogenetic protein signaling protects against cerulein-induced pancreatic fibrosis
Abstract
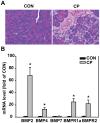
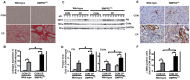
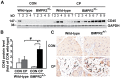
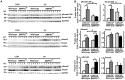
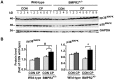
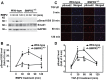
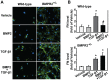
Bone morphogenetic proteins (BMPs) have an anti-fibrogenic function in the kidney, lung, and liver. However, their role in chronic pancreatitis (CP) is unknown. The aim of this study was to define the anti-fibrogenic role of BMP signaling in the pancreas in vivo under CP induction. Mice with a deletion of BMP type II receptor (BMPR2(+/-)) were used in this study in comparison with wild-type mice. CP was induced by repetitive cerulein injection intraperitoneally for 4 weeks, and the severity of CP was evaluated. Pancreatic stellate cells (PSCs) were isolated from the mice and treated with BMP2 and TGF-β in vitro, and extracellular matrix protein (ECM) production was measured. Smad and mitogen-activated protein kinase (MAPK) signaling was also evaluated. BMPR2(+/-) mice revealed a greater pancreatic fibrosis, PSC activation and leukocyte infiltration after CP induction compared to wild-type mice (P<0.05). Under CP induction, phospho (p)Smad1/5/8 was elevated in wild-type mice and this effect was abolished in BMPR2(+/-) mice; pSmad2 and pp38(MAPK) were further enhanced in BMPR2(+/-) mice compared to wild-type mice (P<0.05). In vitro, BMP2 inhibited TGF-β-induced ECM protein fibronectin production in wild-type PSCs; this effect was abolished in BMPR2(+/-) PSCs (P<0.05). In BMPR2(+/-) PSCs, pSmad1/5/8 level was barely detectable upon BMP2 stimulation, while pSmad2 level was further enhanced by TGF-β stimulation, compared to wild-type PSCs (P<0.05). BMPR2/Smad1/5/8 signaling plays a protective role against cerulein-induced pancreatic fibrosis by inhibiting Smad2 and p38(MAPK) signaling pathways.
Conflict of interest statement
Figures
Similar articles
-
BMP2 inhibits TGF-β-induced pancreatic stellate cell activation and extracellular matrix formation.Am J Physiol Gastrointest Liver Physiol. 2013 May 1;304(9):G804-13. doi: 10.1152/ajpgi.00306.2012. Epub 2013 Feb 21. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23429583 Free PMC article.
-
Gremlin is a key pro-fibrogenic factor in chronic pancreatitis.J Mol Med (Berl). 2015 Oct;93(10):1085-1093. doi: 10.1007/s00109-015-1308-9. Epub 2015 Jul 5. J Mol Med (Berl). 2015. PMID: 26141517 Free PMC article.
-
Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling.Pancreas. 2005 Apr;30(3):e71-9. doi: 10.1097/01.mpa.0000157388.54016.0a. Pancreas. 2005. PMID: 15782092
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Emerging role of BMPs/BMPR2 signaling pathway in treatment for pulmonary fibrosis.Biomed Pharmacother. 2024 Sep;178:117178. doi: 10.1016/j.biopha.2024.117178. Epub 2024 Aug 13. Biomed Pharmacother. 2024. PMID: 39142248 Free PMC article. Review.
Cited by
-
Candidate rejuvenating factor GDF11 and tissue fibrosis: friend or foe?Geroscience. 2020 Dec;42(6):1475-1498. doi: 10.1007/s11357-020-00279-w. Epub 2020 Oct 6. Geroscience. 2020. PMID: 33025411 Free PMC article. Review.
-
Opposing Roles of BMP and TGF-β Signaling Pathways in Pancreatitis: Mechanisms and Therapeutic Implication.Adv Res Gastroenterol Hepatol. 2019;13(5):555871. Epub 2019 Sep 4. Adv Res Gastroenterol Hepatol. 2019. PMID: 32211568 Free PMC article.
-
BMP type II receptor as a therapeutic target in pulmonary arterial hypertension.Cell Mol Life Sci. 2017 Aug;74(16):2979-2995. doi: 10.1007/s00018-017-2510-4. Epub 2017 Apr 26. Cell Mol Life Sci. 2017. PMID: 28447104 Free PMC article. Review.
-
Poly(ADP-Ribose) Polymerase 1 Promotes Inflammation and Fibrosis in a Mouse Model of Chronic Pancreatitis.Int J Mol Sci. 2021 Mar 30;22(7):3593. doi: 10.3390/ijms22073593. Int J Mol Sci. 2021. PMID: 33808340 Free PMC article.
-
Comparable Responses in Male and Female Mice to Cerulein-Induced Chronic Pancreatic Injury and Recovery.JOP. 2018 Sep;19(5):236-243. Epub 2018 Sep 28. JOP. 2018. PMID: 30636940 Free PMC article.
References
-
- Shrikhande SV, Martignoni ME, Shrikhande M, Kappeler A, Ramesh H, et al. (2003) Comparison of histological features and inflammatory cell reaction in alcoholic, idiopathic and tropical chronic pancreatitis. Br J Surg 90: 1565–1572. - PubMed
-
- Spanier BW, Dijkgraaf MG, Bruno MJ (2008) Trends and forecasts of hospital admissions for acute and chronic pancreatitis in the Netherlands. Eur J Gastroenterol Hepatol 20: 653–658. - PubMed
-
- Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

