Implication of PMLIV in both intrinsic and innate immunity
- PMID: 24586174
- PMCID: PMC3937294
- DOI: 10.1371/journal.ppat.1003975
Implication of PMLIV in both intrinsic and innate immunity
Abstract
PML/TRIM19, the organizer of nuclear bodies (NBs), has been implicated in the antiviral response to diverse RNA and DNA viruses. Several PML isoforms generated from a single PML gene by alternative splicing, share the same N-terminal region containing the RBCC/tripartite motif but differ in their C-terminal sequences. Recent studies of all the PML isoforms reveal the specific functions of each. The knockout of PML renders mice more sensitive to vesicular stomatitis virus (VSV). Here we report that among PML isoforms (PMLI to PMLVIIb), only PMLIII and PMLIV confer resistance to VSV. Unlike PMLIII, whose anti-VSV activity is IFN-independent, PMLIV can act at two stages: it confers viral resistance directly in an IFN-independent manner and also specifically enhances IFN-β production via a higher activation of IRF3, thus protecting yet uninfected cells from oncoming infection. PMLIV SUMOylation is required for both activities. This demonstrates for the first time that PMLIV is implicated in innate immune response through enhanced IFN-β synthesis. Depletion of IRF3 further demonstrates the dual activity of PMLIV, since it abrogated PMLIV-induced IFN synthesis but not PMLIV-induced inhibition of viral proteins. Mechanistically, PMLIV enhances IFN-β synthesis by regulating the cellular distribution of Pin1 (peptidyl-prolyl cis/trans isomerase), inducing its recruitment to PML NBs where both proteins colocalize. The interaction of SUMOylated PMLIV with endogenous Pin1 and its recruitment within PML NBs prevents the degradation of activated IRF3, and thus potentiates IRF3-dependent production of IFN-β. Whereas the intrinsic antiviral activity of PMLIV is specific to VSV, its effect on IFN-β synthesis is much broader, since it affects a key actor of innate immune pathways. Our results show that, in addition to its intrinsic anti-VSV activity, PMLIV positively regulates IFN-β synthesis in response to different inducers, thus adding PML/TRIM19 to the growing list of TRIM proteins implicated in both intrinsic and innate immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
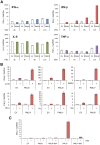
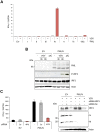
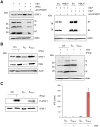
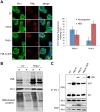

Similar articles
-
Resistance to rabies virus infection conferred by the PMLIV isoform.J Virol. 2010 Oct;84(20):10719-26. doi: 10.1128/JVI.01286-10. Epub 2010 Aug 11. J Virol. 2010. PMID: 20702643 Free PMC article.
-
Promyelocytic leukemia isoform IV confers resistance to encephalomyocarditis virus via the sequestration of 3D polymerase in nuclear bodies.J Virol. 2011 Dec;85(24):13164-73. doi: 10.1128/JVI.05808-11. Epub 2011 Oct 12. J Virol. 2011. PMID: 21994459 Free PMC article.
-
[Implication of PML nuclear bodies in intrinsic and innate immunity].Med Sci (Paris). 2014 Aug-Sep;30(8-9):765-71. doi: 10.1051/medsci/20143008014. Epub 2014 Sep 1. Med Sci (Paris). 2014. PMID: 25174753 Review. French.
-
PML positively regulates interferon gamma signaling.Biochimie. 2011 Mar;93(3):389-98. doi: 10.1016/j.biochi.2010.11.005. Epub 2010 Nov 27. Biochimie. 2011. PMID: 21115099
-
Role of promyelocytic leukemia protein in host antiviral defense.J Interferon Cytokine Res. 2011 Jan;31(1):145-58. doi: 10.1089/jir.2010.0111. Epub 2011 Jan 3. J Interferon Cytokine Res. 2011. PMID: 21198351 Review.
Cited by
-
Promyelocytic Leukemia Protein Isoform II Promotes Transcription Factor Recruitment To Activate Interferon Beta and Interferon-Responsive Gene Expression.Mol Cell Biol. 2015 May;35(10):1660-72. doi: 10.1128/MCB.01478-14. Epub 2015 Mar 2. Mol Cell Biol. 2015. PMID: 25733689 Free PMC article.
-
Autophagic degradation of PML promotes susceptibility to HSV-1 by stress-induced corticosterone.Theranostics. 2020 Jul 11;10(20):9032-9049. doi: 10.7150/thno.46921. eCollection 2020. Theranostics. 2020. PMID: 32802177 Free PMC article.
-
Dual signaling via interferon and DNA damage response elicits entrapment by giant PML nuclear bodies.Elife. 2022 Mar 23;11:e73006. doi: 10.7554/eLife.73006. Elife. 2022. PMID: 35319461 Free PMC article.
-
Porcine promyelocytic leukemia protein isoforms suppress Japanese encephalitis virus replication in PK15 cells.Virol J. 2023 Nov 29;20(1):280. doi: 10.1186/s12985-023-02212-x. Virol J. 2023. PMID: 38031162 Free PMC article.
-
PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx.PLoS Pathog. 2015 Nov 13;11(11):e1005280. doi: 10.1371/journal.ppat.1005280. eCollection 2015 Nov. PLoS Pathog. 2015. PMID: 26566030 Free PMC article.
References
-
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19: 623–655. - PubMed
-
- Chelbi-Alix MK, Wietzerbin J (2007) Interferon, a growing cytokine family: 50 years of interferon research. Biochimie 89 6–7: 713–718. - PubMed
-
- Albertini AA, Ruigrok RW, Blondel D (2011) Rabies virus transcription and replication. Adv Virus Res 79: 1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

