In vivo ablation of type I interferon receptor from cardiomyocytes delays coxsackieviral clearance and accelerates myocardial disease
- PMID: 24574394
- PMCID: PMC3993796
- DOI: 10.1128/JVI.00184-14
In vivo ablation of type I interferon receptor from cardiomyocytes delays coxsackieviral clearance and accelerates myocardial disease
Abstract
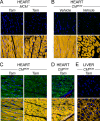
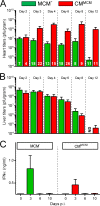
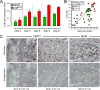
Acute coxsackievirus B3 (CVB3) infection is one of the most prevalent causes of acute myocarditis, a disease that frequently is identified only after the sudden death of apparently healthy individuals. CVB3 infects cardiomyocytes, but the infection is highly focal, even in the absence of a strong adaptive immune response, suggesting that virus spread within the heart may be tightly constrained by the innate immune system. Type I interferons (T1IFNs) are an obvious candidate, and T1IFN receptor (T1IFNR) knockout mice are highly susceptible to CVB3 infection, succumbing within a few days of challenge. Here, we investigated the role of T1IFNs in the heart using a mouse model in which the T1IFNR gene can be ablated in vivo, specifically in cardiomyocytes. We found that T1IFN signaling into cardiomyocytes contributed substantially to the suppression of viral replication and infectious virus yield in the heart; in the absence of such signaling, virus titers were markedly elevated by day 3 postinfection (p.i.) and remained high at day 12 p.i., a time point at which virus was absent from genetically intact littermates, suggesting that the T1IFN-unresponsive cardiomyocytes may act as a safe haven for the virus. Nevertheless, in these mice the myocardial infection remained highly focal, despite the cardiomyocytes' inability to respond to T1IFN, indicating that other factors, as yet unidentified, are sufficient to prevent the more widespread dissemination of the infection throughout the heart. The absence of T1IFN signaling into cardiomyocytes also was accompanied by a profound acceleration and exacerbation of myocarditis and by a significant increase in mortality.
Importance: Acute coxsackievirus B3 (CVB3) infection is one of the most common causes of acute myocarditis, a serious and sometimes fatal disease. To optimize treatment, it is vital that we identify the immune factors that limit virus spread in the heart and other organs. Type I interferons play a key role in controlling many virus infections, but it has been suggested that they may not directly impact CVB3 infection within the heart. Here, using a novel line of transgenic mice, we show that these cytokines signal directly into cardiomyocytes, limiting viral replication, myocarditis, and death.
Figures

Similar articles
-
Ubiquitin-like protein ISG15 (interferon-stimulated gene of 15 kDa) in host defense against heart failure in a mouse model of virus-induced cardiomyopathy.Circulation. 2014 Oct 28;130(18):1589-600. doi: 10.1161/CIRCULATIONAHA.114.009847. Epub 2014 Aug 27. Circulation. 2014. PMID: 25165091
-
Development of a new mouse model for coxsackievirus-induced myocarditis by attenuating coxsackievirus B3 virulence in the pancreas.Cardiovasc Res. 2020 Aug 1;116(10):1756-1766. doi: 10.1093/cvr/cvz259. Cardiovasc Res. 2020. PMID: 31598635
-
CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis.Circ Res. 2009 Mar 13;104(5):628-38. doi: 10.1161/CIRCRESAHA.108.192179. Epub 2009 Jan 22. Circ Res. 2009. PMID: 19168435
-
The role of sex differences in autophagy in the heart during coxsackievirus B3-induced myocarditis.J Cardiovasc Transl Res. 2014 Mar;7(2):182-91. doi: 10.1007/s12265-013-9525-5. Epub 2013 Dec 10. J Cardiovasc Transl Res. 2014. PMID: 24323874 Free PMC article. Review.
-
Manipulating intestinal immunity and microflora: an alternative solution to viral myocarditis?Future Microbiol. 2012 Oct;7(10):1207-16. doi: 10.2217/fmb.12.96. Future Microbiol. 2012. PMID: 23030425 Review.
Cited by
-
Silencing the CSF-1 Axis Using Nanoparticle Encapsulated siRNA Mitigates Viral and Autoimmune Myocarditis.Front Immunol. 2018 Oct 8;9:2303. doi: 10.3389/fimmu.2018.02303. eCollection 2018. Front Immunol. 2018. PMID: 30349538 Free PMC article.
-
The immunoproteasome-specific inhibitor ONX 0914 reverses susceptibility to acute viral myocarditis.EMBO Mol Med. 2018 Feb;10(2):200-218. doi: 10.15252/emmm.201708089. EMBO Mol Med. 2018. PMID: 29295868 Free PMC article.
-
Cleavage of Desmosomal Cadherins Promotes γ-Catenin Degradation and Benefits Wnt Signaling in Coxsackievirus B3-Induced Destruction of Cardiomyocytes.Front Microbiol. 2020 May 8;11:767. doi: 10.3389/fmicb.2020.00767. eCollection 2020. Front Microbiol. 2020. PMID: 32457708 Free PMC article.
-
Development of Group B Coxsackievirus as an Oncolytic Virus: Opportunities and Challenges.Viruses. 2021 Jun 5;13(6):1082. doi: 10.3390/v13061082. Viruses. 2021. PMID: 34198859 Free PMC article.
-
Interferons and interferon-related pathways in heart disease.Front Cardiovasc Med. 2024 Apr 11;11:1357343. doi: 10.3389/fcvm.2024.1357343. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38665231 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

