Dendritic cell-lymphocyte cross talk downregulates host restriction factor SAMHD1 and stimulates HIV-1 replication in dendritic cells
- PMID: 24574390
- PMCID: PMC3993804
- DOI: 10.1128/JVI.03057-13
Dendritic cell-lymphocyte cross talk downregulates host restriction factor SAMHD1 and stimulates HIV-1 replication in dendritic cells
Abstract
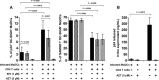
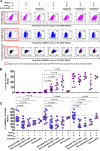
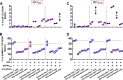
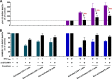
Human immunodeficiency virus type 1 (HIV-1) replication in dendritic cells (DCs) is restricted by SAMHD1. This factor is counteracted by the viral protein Vpx; Vpx is found in HIV-2 and simian immunodeficiency virus (SIV) from sooty mangabeys (SIVsm) or from macaques (SIVmac) but is absent from HIV-1. We previously observed that HIV-1 replication in immature DCs is stimulated by cocultivation with primary T and B lymphocytes, suggesting that HIV-1 restriction in DCs may be overcome under coculture conditions. Here, we aimed to decipher the mechanism of SAMHD1-mediated restriction in DC-lymphocyte coculture. We found that coculture with lymphocytes downregulated SAMHD1 expression and was associated with increased HIV-1 replication in DCs. Moreover, in infected DC-T lymphocyte cocultures, DCs acquired maturation status and secreted type 1 interferon (alpha interferon [IFN-α]). The blockade of DC-lymphocyte cross talk by anti-ICAM-1 antibody markedly inhibited the stimulation of HIV-1 replication and prevented the downregulation of SAMHD1 expression in cocultured DCs. These results demonstrate that, in contrast to purified DCs, cross talk with lymphocytes downregulates SAMHD1 expression in DCs, triggering HIV-1 replication and an antiviral immune response. Therefore, HIV-1 replication and immune sensing by DCs should be investigated in more physiologically relevant models of DC/lymphocyte coculture.
Importance: SAMHD1 restricts HIV-1 replication in dendritic cells (DCs). Here, we demonstrate that, in a coculture model of DCs and lymphocytes mimicking early mucosal HIV-1 infection, stimulation of HIV-1 replication in DCs is associated with downregulation of SAMHD1 expression and activation of innate immune sensing by DCs. We propose that DC-lymphocyte cross talk occurring in vivo modulates host restriction factor SAMHD1, promoting HIV-1 replication in cellular reservoirs and stimulating immune sensing.
Figures



Similar articles
-
SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells.J Virol. 2013 Mar;87(5):2846-56. doi: 10.1128/JVI.02514-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269793 Free PMC article.
-
Interference with SAMHD1 Restores Late Gene Expression of Modified Vaccinia Virus Ankara in Human Dendritic Cells and Abrogates Type I Interferon Expression.J Virol. 2019 Oct 29;93(22):e01097-19. doi: 10.1128/JVI.01097-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462561 Free PMC article.
-
SAMHD1 restricts HIV-1 infection in dendritic cells (DCs) by dNTP depletion, but its expression in DCs and primary CD4+ T-lymphocytes cannot be upregulated by interferons.Retrovirology. 2012 Dec 11;9:105. doi: 10.1186/1742-4690-9-105. Retrovirology. 2012. PMID: 23231760 Free PMC article.
-
SAMHD1 in Retroviral Restriction and Innate Immune Sensing--Should We Leash the Hound?Curr HIV Res. 2016;14(3):225-34. doi: 10.2174/1570162x14999160224102515. Curr HIV Res. 2016. PMID: 26957197 Review.
-
SAMHD1 host restriction factor: a link with innate immune sensing of retrovirus infection.J Mol Biol. 2013 Dec 13;425(24):4981-94. doi: 10.1016/j.jmb.2013.10.022. Epub 2013 Oct 23. J Mol Biol. 2013. PMID: 24161438 Review.
Cited by
-
Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells.PLoS Pathog. 2015 Jun 29;11(6):e1005005. doi: 10.1371/journal.ppat.1005005. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26121641 Free PMC article.
-
New Approaches to Dendritic Cell-Based Therapeutic Vaccines Against HIV-1 Infection.Front Immunol. 2022 Jan 4;12:719664. doi: 10.3389/fimmu.2021.719664. eCollection 2021. Front Immunol. 2022. PMID: 35058917 Free PMC article. Review.
-
Virion-Associated Vpr Alleviates a Postintegration Block to HIV-1 Infection of Dendritic Cells.J Virol. 2017 Jun 9;91(13):e00051-17. doi: 10.1128/JVI.00051-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424288 Free PMC article.
-
SAMHD1 Phosphorylation Coordinates the Anti-HIV-1 Response by Diverse Interferons and Tyrosine Kinase Inhibition.mBio. 2018 May 15;9(3):e00819-18. doi: 10.1128/mBio.00819-18. mBio. 2018. PMID: 29764952 Free PMC article.
-
The Dynamic Interplay between HIV-1, SAMHD1, and the Innate Antiviral Response.Front Immunol. 2017 Nov 10;8:1541. doi: 10.3389/fimmu.2017.01541. eCollection 2017. Front Immunol. 2017. PMID: 29176984 Free PMC article. Review.
References
-
- Berger A, Sommer AF, Zwarg J, Hamdorf M, Welzel K, Esly N, Panitz S, Reuter A, Ramos I, Jatiani A, Mulder LC, Fernandez-Sesma A, Rutsch F, Simon V, Konig R, Flory E. 2011. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutieres syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 7:e1002425. 10.1371/journal.ppat.1002425 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

