Adenovirus vector-induced CD8⁺ T effector memory cell differentiation and recirculation, but not proliferation, are important for protective immunity against experimental Trypanosoma cruzi Infection
- PMID: 24568548
- PMCID: PMC3997096
- DOI: 10.1089/hum.2013.218
Adenovirus vector-induced CD8⁺ T effector memory cell differentiation and recirculation, but not proliferation, are important for protective immunity against experimental Trypanosoma cruzi Infection
Abstract
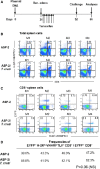
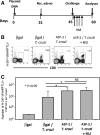
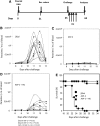
Heterologous prime-boost vaccination using plasmid DNA followed by replication-defective adenovirus vector generates a large number of specific CD8⁺ T effector memory (TEM) cells that provide long-term immunity against a variety of pathogens. In the present study, we initially characterized the frequency, phenotype, and function of these T cells in vaccinated mice that were subjected to infectious challenge with the human protozoan parasite Trypanosoma cruzi. We observed that the frequency of the specific CD8⁺ T cells in the spleens of the vaccinated mice increased after challenge. Specific TEM cells differentiated into cells with a KLRG1(High) CD27(Low) CD43(Low) CD183(Low)T-bet(High) Eomes(Low) phenotype and capable to produce simultaneously the antiparasitic mediators IFNγ and TNF. Using the gzmBCreERT2/ROSA26EYFP transgenic mouse line, in which the cells that express Granzyme B after immunization, are indelibly labeled with enhanced yellow fluorescent protein, we confirmed that CD8⁺ T cells present after challenge were indeed TEM cells that had been induced by vaccination. Subsequently, we observed that the in vivo increase in the frequency of the specific CD8⁺ T cells was not because of an anamnestic immune response. Most importantly, after challenge, the increase in the frequency of specific cells and the protective immunity they mediate were insensitive to treatment with the cytostatic toxic agent hydroxyurea. We have previously described that the administration of the drug FTY720, which reduces lymphocyte recirculation, severely impairs protective immunity, and our evidence supports the model that when large amounts of antigen-experienced CD8⁺ TEM cells are present after heterologous prime-boost vaccination, differentiation, and recirculation, rather than proliferation, are key for the resultant protective immunity.
Figures




Similar articles
-
Heterologous plasmid DNA prime-recombinant human adenovirus 5 boost vaccination generates a stable pool of protective long-lived CD8(+) T effector memory cells specific for a human parasite, Trypanosoma cruzi.Infect Immun. 2011 May;79(5):2120-30. doi: 10.1128/IAI.01190-10. Epub 2011 Feb 28. Infect Immun. 2011. PMID: 21357719 Free PMC article.
-
Perforin and gamma interferon expression are required for CD4+ and CD8+ T-cell-dependent protective immunity against a human parasite, Trypanosoma cruzi, elicited by heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination.Infect Immun. 2009 Oct;77(10):4383-95. doi: 10.1128/IAI.01459-08. Epub 2009 Aug 3. Infect Immun. 2009. PMID: 19651871 Free PMC article.
-
Vaccination using recombinants influenza and adenoviruses encoding amastigote surface protein-2 are highly effective on protection against Trypanosoma cruzi infection.PLoS One. 2013 Apr 24;8(4):e61795. doi: 10.1371/journal.pone.0061795. Print 2013. PLoS One. 2013. PMID: 23637908 Free PMC article.
-
CD8+ T cells in Trypanosoma cruzi infection.Semin Immunopathol. 2015 May;37(3):233-8. doi: 10.1007/s00281-015-0481-9. Epub 2015 Apr 29. Semin Immunopathol. 2015. PMID: 25921214 Free PMC article. Review.
-
Trypanosoma cruzi infection from the view of CD8+ T cell immunity--an infection model for developing T cell vaccine.Parasitol Int. 2008 Mar;57(1):38-48. doi: 10.1016/j.parint.2007.07.005. Epub 2007 Aug 1. Parasitol Int. 2008. PMID: 17728174 Review.
Cited by
-
Evaluation of pathogen P21 protein as a potential modulator of the protective immunity induced by Trypanosoma cruzi attenuated parasites.Mem Inst Oswaldo Cruz. 2019;114:e180571. doi: 10.1590/0074-02760180571. Epub 2019 May 20. Mem Inst Oswaldo Cruz. 2019. PMID: 31116244 Free PMC article.
-
Unconventional Pro-inflammatory CD4+ T Cell Response in B Cell-Deficient Mice Infected with Trypanosoma cruzi.Front Immunol. 2017 Nov 21;8:1548. doi: 10.3389/fimmu.2017.01548. eCollection 2017. Front Immunol. 2017. PMID: 29209313 Free PMC article.
-
Down Modulation of Host Immune Response by Amino Acid Repeats Present in a Trypanosoma cruzi Ribosomal Antigen.Front Microbiol. 2017 Nov 10;8:2188. doi: 10.3389/fmicb.2017.02188. eCollection 2017. Front Microbiol. 2017. PMID: 29176965 Free PMC article.
-
Drug-cured experimental Trypanosoma cruzi infections confer long-lasting and cross-strain protection.PLoS Negl Trop Dis. 2020 Apr 17;14(4):e0007717. doi: 10.1371/journal.pntd.0007717. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32302312 Free PMC article.
-
Genetic vaccination against experimental infection with myotropic parasite strains of Trypanosoma cruzi.Mediators Inflamm. 2014;2014:605023. doi: 10.1155/2014/605023. Epub 2014 Jun 26. Mediators Inflamm. 2014. PMID: 25061263 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

