B-cell responses to pregnancy-restricted and -unrestricted Plasmodium falciparum erythrocyte membrane protein 1 antigens in Ghanaian women naturally exposed to malaria parasites
- PMID: 24566620
- PMCID: PMC3993431
- DOI: 10.1128/IAI.01514-13
B-cell responses to pregnancy-restricted and -unrestricted Plasmodium falciparum erythrocyte membrane protein 1 antigens in Ghanaian women naturally exposed to malaria parasites
Abstract
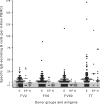
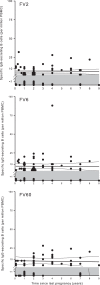
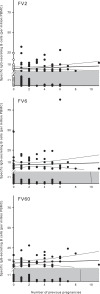
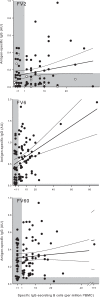
Protective immunity to Plasmodium falciparum malaria acquired after natural exposure is largely antibody mediated. IgG-specific P. falciparum EMP1 (PfEMP1) proteins on the infected erythrocyte surface are particularly important. The transient antibody responses and the slowly acquired protective immunity probably reflect the clonal antigenic variation and allelic polymorphism of PfEMP1. However, it is likely that other immune-evasive mechanisms are also involved, such as interference with formation and maintenance of immunological memory. We measured PfEMP1-specific antibody levels by enzyme-linked immunosorbent assay (ELISA) and memory B-cell frequencies by enzyme-linked immunosorbent spot (ELISPOT) assay in a cohort of P. falciparum-exposed nonpregnant Ghanaian women. The antigens used were a VAR2CSA-type PfEMP1 (IT4VAR04) with expression restricted to parasites infecting the placenta, as well as two commonly recognized PfEMP1 proteins (HB3VAR06 and IT4VAR60) implicated in rosetting and not pregnancy restricted. This enabled, for the first time, a direct comparison in the same individuals of immune responses specific for a clinically important parasite antigen expressed only during well-defined periods (pregnancy) to responses specific for comparable antigens expressed independent of pregnancy. Our data indicate that PfEMP1-specific B-cell memory is adequately acquired even when antigen exposure is infrequent (e.g., VAR2CSA-type PfEMP1). Furthermore, immunological memory specific for VAR2CSA-type PfEMP1 can be maintained for many years without antigen reexposure and after circulating antigen-specific IgG has disappeared. The study provides evidence that natural exposure to P. falciparum leads to formation of durable B-cell immunity to clinically important PfEMP1 antigens. This has encouraging implications for current efforts to develop PfEMP1-based vaccines.
Figures
Similar articles
-
Kinetics of B cell responses to Plasmodium falciparum erythrocyte membrane protein 1 in Ghanaian women naturally exposed to malaria parasites.J Immunol. 2014 Jun 1;192(11):5236-44. doi: 10.4049/jimmunol.1400325. Epub 2014 Apr 23. J Immunol. 2014. PMID: 24760153 Free PMC article. Clinical Trial.
-
Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1.Proc Natl Acad Sci U S A. 2011 Jul 26;108(30):12485-90. doi: 10.1073/pnas.1103708108. Epub 2011 Jul 11. Proc Natl Acad Sci U S A. 2011. PMID: 21746929 Free PMC article.
-
Plasmodium falciparum: VAR2CSA expressed during pregnancy-associated malaria is partially resistant to proteolytic cleavage by trypsin.Exp Parasitol. 2007 Sep;117(1):1-8. doi: 10.1016/j.exppara.2007.03.002. Epub 2007 Mar 14. Exp Parasitol. 2007. PMID: 17442305
-
VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria.Parasitology. 2007;134(Pt 13):1871-6. doi: 10.1017/S0031182007000121. Parasitology. 2007. PMID: 17958922 Review.
-
VAR2CSA-Mediated Host Defense Evasion of Plasmodium falciparum Infected Erythrocytes in Placental Malaria.Front Immunol. 2021 Feb 9;11:624126. doi: 10.3389/fimmu.2020.624126. eCollection 2020. Front Immunol. 2021. PMID: 33633743 Free PMC article. Review.
Cited by
-
Malaria and immunity during pregnancy and postpartum: a tale of two species.Parasitology. 2015 Jul;142(8):999-1015. doi: 10.1017/S0031182015000074. Epub 2015 Mar 3. Parasitology. 2015. PMID: 25731914 Free PMC article. Review.
-
Antibody levels to recombinant VAR2CSA domains vary with Plasmodium falciparum parasitaemia, gestational age, and gravidity, but do not predict pregnancy outcomes.Malar J. 2018 Mar 9;17(1):106. doi: 10.1186/s12936-018-2258-9. Malar J. 2018. PMID: 29523137 Free PMC article.
-
Antibody responses to Plasmodium falciparum and Plasmodium vivax blood-stage and sporozoite antigens in the postpartum period.Sci Rep. 2016 Aug 25;6:32159. doi: 10.1038/srep32159. Sci Rep. 2016. PMID: 27558000 Free PMC article.
-
IgG Responses to the Plasmodium falciparum Antigen VAR2CSA in Colombia Are Restricted to Pregnancy and Are Not Induced by Exposure to Plasmodium vivax.Infect Immun. 2018 Jul 23;86(8):e00136-18. doi: 10.1128/IAI.00136-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29784859 Free PMC article.
-
α2-Macroglobulin Can Crosslink Multiple Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes.PLoS Pathog. 2015 Jul 2;11(7):e1005022. doi: 10.1371/journal.ppat.1005022. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26134405 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

