Polarizable empirical force field for hexopyranose monosaccharides based on the classical Drude oscillator
- PMID: 24564643
- PMCID: PMC4143499
- DOI: 10.1021/jp412696m
Polarizable empirical force field for hexopyranose monosaccharides based on the classical Drude oscillator
Abstract
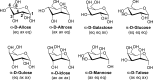
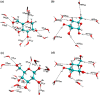
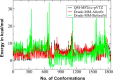
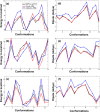
A polarizable empirical force field based on the classical Drude oscillator is presented for the hexopyranose form of selected monosaccharides. Parameter optimization targeted quantum mechanical (QM) dipole moments, solute-water interaction energies, vibrational frequencies, and conformational energies. Validation of the model was based on experimental data on crystals, densities of aqueous-sugar solutions, diffusion constants of glucose, and rotational preferences of the exocylic hydroxymethyl of d-glucose and d-galactose in aqueous solution as well as additional QM data. Notably, the final model involves a single electrostatic model for all sixteen diastereomers of the monosaccharides, indicating the transferability of the polarizable model. The presented parameters are anticipated to lay the foundation for a comprehensive polarizable force field for saccharides that will be compatible with the polarizable Drude parameters for lipids and proteins, allowing for simulations of glycolipids and glycoproteins.
Figures
Similar articles
-
CHARMM Drude Polarizable Force Field for Glycosidic Linkages Involving Pyranoses and Furanoses.J Chem Theory Comput. 2018 Jun 12;14(6):3132-3143. doi: 10.1021/acs.jctc.8b00175. Epub 2018 May 4. J Chem Theory Comput. 2018. PMID: 29694037 Free PMC article.
-
An Empirical Polarizable Force Field Based on the Classical Drude Oscillator Model: Development History and Recent Applications.Chem Rev. 2016 May 11;116(9):4983-5013. doi: 10.1021/acs.chemrev.5b00505. Epub 2016 Jan 27. Chem Rev. 2016. PMID: 26815602 Free PMC article. Review.
-
Additive empirical force field for hexopyranose monosaccharides.J Comput Chem. 2008 Nov 30;29(15):2543-64. doi: 10.1002/jcc.21004. J Comput Chem. 2008. PMID: 18470966 Free PMC article.
-
Refinement of the Drude Polarizable Force Field for Hexose Monosaccharides: Capturing Ring Conformational Dynamics with Enhanced Accuracy.J Chem Theory Comput. 2024 Oct 22;20(20):9161-9177. doi: 10.1021/acs.jctc.4c00656. Epub 2024 Oct 9. J Chem Theory Comput. 2024. PMID: 39383338
-
Recent Advances in Polarizable Force Fields for Macromolecules: Microsecond Simulations of Proteins Using the Classical Drude Oscillator Model.J Phys Chem Lett. 2014 Sep 18;5(18):3144-3150. doi: 10.1021/jz501315h. Epub 2014 Aug 27. J Phys Chem Lett. 2014. PMID: 25247054 Free PMC article. Review.
Cited by
-
Three-Dimensional Structures of Carbohydrates and Where to Find Them.Int J Mol Sci. 2020 Oct 18;21(20):7702. doi: 10.3390/ijms21207702. Int J Mol Sci. 2020. PMID: 33081008 Free PMC article. Review.
-
CHARMM Drude Polarizable Force Field for Glycosidic Linkages Involving Pyranoses and Furanoses.J Chem Theory Comput. 2018 Jun 12;14(6):3132-3143. doi: 10.1021/acs.jctc.8b00175. Epub 2018 May 4. J Chem Theory Comput. 2018. PMID: 29694037 Free PMC article.
-
An Empirical Polarizable Force Field Based on the Classical Drude Oscillator Model: Development History and Recent Applications.Chem Rev. 2016 May 11;116(9):4983-5013. doi: 10.1021/acs.chemrev.5b00505. Epub 2016 Jan 27. Chem Rev. 2016. PMID: 26815602 Free PMC article. Review.
-
Effects of varying the 6-position oxidation state of hexopyranoses: a systematic comparative computational analysis of 48 monosaccharide stereoisomers.J Mol Model. 2017 Jul;23(7):214. doi: 10.1007/s00894-017-3385-x. Epub 2017 Jun 27. J Mol Model. 2017. PMID: 28656484 Free PMC article.
-
Conformational Heterogeneity of the HIV Envelope Glycan Shield.Sci Rep. 2017 Jun 30;7(1):4435. doi: 10.1038/s41598-017-04532-9. Sci Rep. 2017. PMID: 28667249 Free PMC article.
References
-
- Dwek R. A.; Butters T. D. Introduction: Glycobiology—Understanding the Language and Meaning of Carbohydrates. Chem. Rev. 2002, 102, 283–284.
-
- Capon B. Mechanism in Carbohydrate Chemistry. Chem. Rev. 1969, 69, 407–498.
-
- Pigman W.The Carbohydrates. Chemistry and Biochemistry, 2nd ed.; Academic Press: New York, 1972; Vol. 1A.
-
- El Kadib A.; Bousmina M. Chitosan Bio-Based Organic–Inorganic Hybrid Aerogel Microspheres. Chem.—Eur. J. 2012, 18, 8264–8277. - PubMed
-
- Koutsopoulos S. Molecular Fabrications of Smart Nanobiomaterials and Applications in Personalized Medicine. Adv. Drug. Delivery Rev. 2012, 64, 1459–1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

