In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer's disease
- PMID: 24563598
- PMCID: PMC3930111
- DOI: 10.1016/j.jyp.2013.11.005
In silico QSAR analysis of quercetin reveals its potential as therapeutic drug for Alzheimer's disease
Abstract
Acetylcholine-esterase (AchE) inhibitors are one of the most potent drug molecules against Alzheimer's disease (AD). But, patients treated with current AchE inhibitors often experience severe side effects. Quercetin is a plant flavonoid compound which can act as AchE inhibitor and it may be a better alternative to current AchE inhibitors in terms of effectiveness with no or fewer side effects.
Aims: The aim of the study was to compare quercetin with conventional AchE inhibitors to search for a better drug candidate.
Methods and materials: Physico-chemical properties of conventional drugs and quercetin were predicted using bioinformatics tools. Molecular docking of these compounds on the active site of AchE was performed using AutoDock and comparative analysis was performed. Later, modification on the basic structure of quercetin with different functional groups was done to perform QSAR analysis.
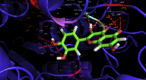
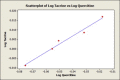
Result and discussion: Quercetin showed a similar drug likeness score to the conventional drugs. The binding strength for quercetin in the active site of the enzyme was -8.8 kcal/mol, which was considerably higher than binding scores for some of the drugs such as donepezil (binding score -7.9 kcal/mol). Fifteen hydrogen bonds were predicted between quercetin and the enzyme whereas conventional drugs had fewer or even no hydrogen bonds. It implies that quercetin can act as a better inhibitor than conventional drugs. To find out even better inhibitor, similar structures of quercetin were searched through SIMCOMP database and a methylation in the 4-OH position of the molecule showed better binding affinity than parent quercetin. Quantitative structure activity relationship study indicated that O-4 methylation was specifically responsible for better affinity.
Conclusion: This in silico study has conclusively predicted the superiority of the natural compound quercetin over the conventional drugs as AchE inhibitor and it sets the need for further in-vitro study of this compound in future.
Keywords: Alzheimer's disease; Cholinesterase inhibitors; In silico; Molecular docking; Quercetin.
Figures

Similar articles
-
De-novo Drug Design, Molecular Docking and In-Silico Molecular Prediction of AChEI Analogues through CADD Approaches as Anti-Alzheimer's Agents.Curr Comput Aided Drug Des. 2020;16(1):54-72. doi: 10.2174/1573409915666190301124210. Curr Comput Aided Drug Des. 2020. PMID: 30827255 Free PMC article.
-
6-Methyluracil derivatives as acetylcholinesterase inhibitors for treatment of Alzheimer's disease.Int J Risk Saf Med. 2015;27 Suppl 1:S69-71. doi: 10.3233/JRS-150694. Int J Risk Saf Med. 2015. PMID: 26639718
-
An In Silico Study Based on QSAR and Molecular Docking and Molecular Dynamics Simulation for the Discovery of Novel Potent Inhibitor against AChE.Pharmaceuticals (Basel). 2024 Jun 25;17(7):830. doi: 10.3390/ph17070830. Pharmaceuticals (Basel). 2024. PMID: 39065681 Free PMC article.
-
An overview of the current and novel drugs for Alzheimer's disease with particular reference to anti-cholinesterase compounds.Curr Pharm Des. 2004;10(25):3121-30. doi: 10.2174/1381612043383359. Curr Pharm Des. 2004. PMID: 15544502 Review.
-
Cholinesterase Inhibitory Activity of Some semi-Rigid Spiro Heterocycles: POM Analyses and Crystalline Structure of Pharmacophore Site.Mini Rev Med Chem. 2018;18(8):711-716. doi: 10.2174/1389557517666170713114039. Mini Rev Med Chem. 2018. PMID: 28714400 Review.
Cited by
-
Strychnos alkaloids: total synthesis, characterization, DFT investigations, and molecular docking with AChE, BuChE, and HSA.Heliyon. 2022 Dec 6;8(12):e11990. doi: 10.1016/j.heliyon.2022.e11990. eCollection 2022 Dec. Heliyon. 2022. PMID: 36531635 Free PMC article.
-
Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health.ACS Omega. 2020 May 14;5(20):11849-11872. doi: 10.1021/acsomega.0c01818. eCollection 2020 May 26. ACS Omega. 2020. PMID: 32478277 Free PMC article.
-
Alzheimer: A Decade of Drug Design. Why Molecular Topology can be an Extra Edge?Curr Neuropharmacol. 2018;16(6):849-864. doi: 10.2174/1570159X15666171129102042. Curr Neuropharmacol. 2018. PMID: 29189164 Free PMC article. Review.
-
Effects of Quercetin and Resveratrol on Zinc Chloride- and Sodium Metavanadate-Induced Passive Avoidance Memory Retention Deficits in Male Mice.Prev Nutr Food Sci. 2021 Mar 31;26(1):67-74. doi: 10.3746/pnf.2021.26.1.67. Prev Nutr Food Sci. 2021. PMID: 33859961 Free PMC article.
-
Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP.Sci Rep. 2019 May 3;9(1):6876. doi: 10.1038/s41598-019-43345-w. Sci Rep. 2019. PMID: 31053743 Free PMC article.
References
-
- Querfurth H.W., LaFerla F.M. Mechanisms of disease. N Engl J Med. 2010;362:329–344. - PubMed
-
- Martins I.J., Hone E., Foster J.K. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–736. - PubMed
-
- Fewlass D.C., Noboa K., Pi-Sunyer F.X., Johnston J.M., Yan S.D., Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Aβ. FASEB J. 2004;18:1870–1878. - PubMed
-
- Profenno L.A., Porsteinsson A.P., Faraone S.V. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

