TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation
- PMID: 24562310
- PMCID: PMC4358322
- DOI: 10.1038/ni.2833
TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKɛ supports the anabolic demands of dendritic cell activation
Abstract
The ligation of Toll-like receptors (TLRs) leads to rapid activation of dendritic cells (DCs). However, the metabolic requirements that support this process remain poorly defined. We found that DC glycolytic flux increased within minutes of exposure to TLR agonists and that this served an essential role in supporting the de novo synthesis of fatty acids for the expansion of the endoplasmic reticulum and Golgi required for the production and secretion of proteins that are integral to DC activation. Signaling via the kinases TBK1, IKKɛ and Akt was essential for the TLR-induced increase in glycolysis by promoting the association of the glycolytic enzyme HK-II with mitochondria. In summary, we identified the rapid induction of glycolysis as an integral component of TLR signaling that is essential for the anabolic demands of the activation and function of DCs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


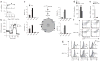

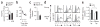

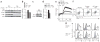

Comment in
-
Glycolytic reprogramming by TLRs in dendritic cells.Nat Immunol. 2014 Apr;15(4):314-5. doi: 10.1038/ni.2852. Nat Immunol. 2014. PMID: 24646590 No abstract available.
-
Dendritic cells: TLR agonists trigger rapid metabolic changes.Nat Rev Immunol. 2014 Apr;14(4):209. doi: 10.1038/nri3652. Nat Rev Immunol. 2014. PMID: 24662378 No abstract available.
-
Translating glycolytic metabolism to innate immunity in dendritic cells.Cell Metab. 2014 May 6;19(5):737-739. doi: 10.1016/j.cmet.2014.04.012. Cell Metab. 2014. PMID: 24807219 Free PMC article.
Similar articles
-
Syk-dependent glycolytic reprogramming in dendritic cells regulates IL-1β production to β-glucan ligands in a TLR-independent manner.J Leukoc Biol. 2019 Dec;106(6):1325-1335. doi: 10.1002/JLB.3A0819-207RR. Epub 2019 Sep 11. J Leukoc Biol. 2019. PMID: 31509298 Free PMC article.
-
FcγR-TLR Cross-Talk Enhances TNF Production by Human Monocyte-Derived DCs via IRF5-Dependent Gene Transcription and Glycolytic Reprogramming.Front Immunol. 2019 Apr 8;10:739. doi: 10.3389/fimmu.2019.00739. eCollection 2019. Front Immunol. 2019. PMID: 31024565 Free PMC article.
-
Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation.Blood. 2010 Jun 10;115(23):4742-9. doi: 10.1182/blood-2009-10-249540. Epub 2010 Mar 29. Blood. 2010. PMID: 20351312 Free PMC article.
-
The role of nitric oxide in metabolic regulation of Dendritic cell immune function.Cancer Lett. 2018 Jan 1;412:236-242. doi: 10.1016/j.canlet.2017.10.032. Epub 2017 Oct 26. Cancer Lett. 2018. PMID: 29107106 Free PMC article. Review.
-
Fueling immunity: insights into metabolism and lymphocyte function.Science. 2013 Oct 11;342(6155):1242454. doi: 10.1126/science.1242454. Science. 2013. PMID: 24115444 Free PMC article. Review.
Cited by
-
Emerging nanomaterials targeting macrophage adapted to abnormal metabolism in cancer and atherosclerosis therapy (Review).Int J Mol Med. 2024 Feb;53(2):13. doi: 10.3892/ijmm.2023.5337. Epub 2023 Dec 8. Int J Mol Med. 2024. PMID: 38063240 Free PMC article. Review.
-
mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation.Cell Rep. 2015 Jul 7;12(1):102-115. doi: 10.1016/j.celrep.2015.05.046. Epub 2015 Jun 25. Cell Rep. 2015. Retraction in: Cell Rep. 2023 Jun 27;42(6):112639. doi: 10.1016/j.celrep.2023.112639. PMID: 26119735 Free PMC article. Retracted.
-
IL-1 induces mitochondrial translocation of IRAK2 to suppress oxidative metabolism in adipocytes.Nat Immunol. 2020 Oct;21(10):1219-1231. doi: 10.1038/s41590-020-0750-1. Epub 2020 Aug 10. Nat Immunol. 2020. PMID: 32778760 Free PMC article.
-
Lipid Metabolism and Tumor Antigen Presentation.Adv Exp Med Biol. 2021;1316:169-189. doi: 10.1007/978-981-33-6785-2_11. Adv Exp Med Biol. 2021. PMID: 33740250
-
Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation.Immunity. 2016 Oct 18;45(4):817-830. doi: 10.1016/j.immuni.2016.09.016. Immunity. 2016. PMID: 27760338 Free PMC article.
References
-
- Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. - PubMed
-
- Jantsch J, et al. Hypoxia and hypoxia-inducible factor-1α modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

