Palmitoylation of δ-catenin by DHHC5 mediates activity-induced synapse plasticity
- PMID: 24562000
- PMCID: PMC5025286
- DOI: 10.1038/nn.3657
Palmitoylation of δ-catenin by DHHC5 mediates activity-induced synapse plasticity
Abstract
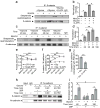
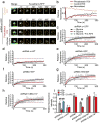
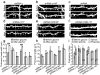
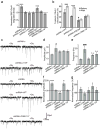
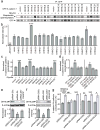
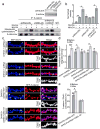
Synaptic cadherin adhesion complexes are known to be key regulators of synapse plasticity. However, the molecular mechanisms that coordinate activity-induced modifications in cadherin localization and adhesion and the subsequent changes in synapse morphology and efficacy remain unknown. We demonstrate that the intracellular cadherin binding protein δ-catenin is transiently palmitoylated by DHHC5 after enhanced synaptic activity and that palmitoylation increases δ-catenin-cadherin interactions at synapses. Both the palmitoylation of δ-catenin and its binding to cadherin are required for activity-induced stabilization of N-cadherin at synapses and the enlargement of postsynaptic spines, as well as the insertion of GluA1 and GluA2 subunits into the synaptic membrane and the concomitant increase in miniature excitatory postsynaptic current amplitude. Notably, context-dependent fear conditioning in mice resulted in increased δ-catenin palmitoylation, as well as increased δ-catenin-cadherin associations at hippocampal synapses. Together these findings suggest a role for palmitoylated δ-catenin in coordinating activity-dependent changes in synaptic adhesion molecules, synapse structure and receptor localization that are involved in memory formation.
Figures


Similar articles
-
Activity-regulated trafficking of the palmitoyl-acyl transferase DHHC5.Nat Commun. 2015 Sep 3;6:8200. doi: 10.1038/ncomms9200. Nat Commun. 2015. PMID: 26334723 Free PMC article.
-
Linkage of N-cadherin to multiple cytoskeletal elements revealed by a proteomic approach in hippocampal neurons.Neurochem Int. 2012 Jul;61(2):240-50. doi: 10.1016/j.neuint.2012.05.008. Epub 2012 May 17. Neurochem Int. 2012. PMID: 22609377
-
N-cadherin regulates molecular organization of excitatory and inhibitory synaptic circuits in adult hippocampus in vivo.Hippocampus. 2014 Aug;24(8):943-962. doi: 10.1002/hipo.22282. Epub 2014 Apr 29. Hippocampus. 2014. PMID: 24753442 Free PMC article.
-
Cadherins and catenins in dendrite and synapse morphogenesis.Cell Adh Migr. 2015;9(3):202-13. doi: 10.4161/19336918.2014.994919. Cell Adh Migr. 2015. PMID: 25914083 Free PMC article. Review.
-
Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity.Trends Neurosci. 2008 Sep;31(9):487-94. doi: 10.1016/j.tins.2008.07.001. Epub 2008 Aug 4. Trends Neurosci. 2008. PMID: 18684518 Free PMC article. Review.
Cited by
-
Saturated free fatty acids and association with memory formation.Nat Commun. 2021 Jun 8;12(1):3443. doi: 10.1038/s41467-021-23840-3. Nat Commun. 2021. PMID: 34103527 Free PMC article.
-
Palmitoylation of LIM Kinase-1 ensures spine-specific actin polymerization and morphological plasticity.Elife. 2015 Apr 17;4:e06327. doi: 10.7554/eLife.06327. Elife. 2015. PMID: 25884247 Free PMC article.
-
Disease-associated synaptic scaffold protein CNK2 modulates PSD size and influences localisation of the regulatory kinase TNIK.Sci Rep. 2020 Mar 31;10(1):5709. doi: 10.1038/s41598-020-62207-4. Sci Rep. 2020. PMID: 32235845 Free PMC article.
-
Mechanisms and functions of protein S-acylation.Nat Rev Mol Cell Biol. 2024 Jun;25(6):488-509. doi: 10.1038/s41580-024-00700-8. Epub 2024 Feb 14. Nat Rev Mol Cell Biol. 2024. PMID: 38355760 Review.
-
Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies.Chem Rev. 2018 Feb 14;118(3):919-988. doi: 10.1021/acs.chemrev.6b00750. Epub 2018 Jan 2. Chem Rev. 2018. PMID: 29292991 Free PMC article.
References
-
- Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. - PubMed
-
- Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. - PubMed
-
- Tang L, Hung CP, Schuman EM. A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron. 1998;20:1165–1175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

