CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis
- PMID: 24561613
- PMCID: PMC4216733
- DOI: 10.1136/gutjnl-2013-306294
CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis
Abstract
Objectives: In chronic liver injury, angiogenesis, the formation of new blood vessels from pre-existing ones, may contribute to progressive hepatic fibrosis and to development of hepatocellular carcinoma. Although hypoxia-induced expression of vascular endothelial growth factor (VEGF) occurs in advanced fibrosis, we hypothesised that inflammation may endorse hepatic angiogenesis already at early stages of fibrosis.
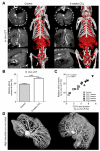
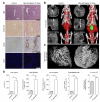
Design: Angiogenesis in livers of c57BL/6 mice upon carbon tetrachloride- or bile duct ligation-induced chronic hepatic injury was non-invasively monitored using in vivo contrast-enhanced micro computed tomography (µCT) and ex vivo anatomical µCT after hepatic Microfil perfusion. Functional contributions of monocyte-derived macrophage subsets for angiogenesis were explored by pharmacological inhibition of CCL2 using the Spiegelmer mNOX-E36.
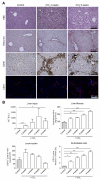
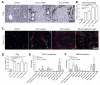
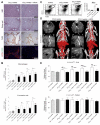
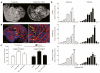
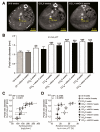
Results: Contrast-enhanced in vivo µCT imaging allowed non-invasive monitoring of the close correlation of angiogenesis, reflected by functional hepatic blood vessel expansion, with experimental fibrosis progression. On a cellular level, inflammatory monocyte-derived macrophages massively accumulated in injured livers, colocalised with newly formed vessels in portal tracts and exhibited pro-angiogenic gene profiles including upregulated VEGF and MMP9. Functional in vivo and anatomical ex vivo µCT analyses demonstrated that inhibition of monocyte infiltration by targeting the chemokine CCL2 prevented fibrosis-associated angiogenesis, but not fibrosis progression. Monocyte-derived macrophages primarily fostered sprouting angiogenesis within the portal vein tract. Portal vein diameter as a measure of portal hypertension depended on fibrosis, but not on angiogenesis.
Conclusions: Inflammation-associated angiogenesis is promoted by CCL2-dependent monocytes during fibrosis progression. Innovative in vivo µCT methodology can accurately monitor angiogenesis and antiangiogenic therapy effects in experimental liver fibrosis.
Keywords: ANGIOGENESIS; CHEMOKINES; COMPUTER TOMOGRAPHY; FIBROSIS; MACROPHAGES.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Comment in
-
CCL2: a link between hepatic inflammation, fibrosis and angiogenesis?Gut. 2014 Dec;63(12):1834-5. doi: 10.1136/gutjnl-2014-306905. Epub 2014 Apr 2. Gut. 2014. PMID: 24694577 No abstract available.
Similar articles
-
Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury.Gut. 2012 Mar;61(3):416-26. doi: 10.1136/gutjnl-2011-300304. Epub 2011 Aug 3. Gut. 2012. PMID: 21813474
-
Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice.Hepatology. 2014 Mar;59(3):1060-72. doi: 10.1002/hep.26783. Epub 2014 Jan 30. Hepatology. 2014. PMID: 24481979
-
The CCR2+ Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers.Cell Mol Gastroenterol Hepatol. 2019;7(2):371-390. doi: 10.1016/j.jcmgh.2018.10.007. Epub 2018 Oct 18. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30704985 Free PMC article.
-
Macrophage heterogeneity in liver injury and fibrosis.J Hepatol. 2014 May;60(5):1090-6. doi: 10.1016/j.jhep.2013.12.025. Epub 2014 Jan 8. J Hepatol. 2014. PMID: 24412603 Review.
-
Monocytes and macrophages as cellular targets in liver fibrosis.Inflamm Allergy Drug Targets. 2009 Sep;8(4):307-18. doi: 10.2174/187152809789352230. Inflamm Allergy Drug Targets. 2009. PMID: 19534673 Review.
Cited by
-
Hepatic macrophages in liver fibrosis: pathogenesis and potential therapeutic targets.BMJ Open Gastroenterol. 2016 May 25;3(1):e000079. doi: 10.1136/bmjgast-2016-000079. eCollection 2016. BMJ Open Gastroenterol. 2016. PMID: 27252881 Free PMC article.
-
Improving In Vivo High-Resolution CT Imaging of the Tumour Vasculature in Xenograft Mouse Models through Reduction of Motion and Bone-Streak Artefacts.PLoS One. 2015 Jun 5;10(6):e0128537. doi: 10.1371/journal.pone.0128537. eCollection 2015. PLoS One. 2015. PMID: 26046526 Free PMC article.
-
Biological functions of macrophage-derived Wnt5a, and its roles in human diseases.Oncotarget. 2016 Oct 11;7(41):67674-67684. doi: 10.18632/oncotarget.11874. Oncotarget. 2016. PMID: 27608847 Free PMC article. Review.
-
Inhibition of intrahepatic monocyte recruitment by Cenicriviroc and extracellular matrix degradation by MMP1 synergistically attenuate liver inflammation and fibrogenesis in vivo.Sci Rep. 2024 Jul 23;14(1):16897. doi: 10.1038/s41598-024-67926-6. Sci Rep. 2024. PMID: 39043893 Free PMC article.
-
Multiscale reconstruction of various vessels in the intact murine liver lobe.Commun Biol. 2022 Mar 24;5(1):260. doi: 10.1038/s42003-022-03221-2. Commun Biol. 2022. PMID: 35332265 Free PMC article.
References
-
- Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–21. - PubMed
-
- Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–95. - PubMed
-
- Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–26. - PubMed
References for Supplementary Methods
-
- Bartneck M, Ritz T, Keul HA, Wambach M, Bornemann J, Gbureck U, et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano. 2012;6:8767–77. - PubMed
-
- Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, et al. Use of the mouse aortic ring assay to study angiogenesis. Nature protocols. 2011;7:89–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
