Megalencephalic leukoencephalopathy with subcortical cysts protein-1 modulates endosomal pH and protein trafficking in astrocytes: relevance to MLC disease pathogenesis
- PMID: 24561067
- PMCID: PMC4003525
- DOI: 10.1016/j.nbd.2014.02.003
Megalencephalic leukoencephalopathy with subcortical cysts protein-1 modulates endosomal pH and protein trafficking in astrocytes: relevance to MLC disease pathogenesis
Abstract
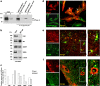
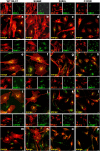
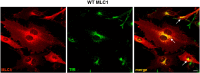
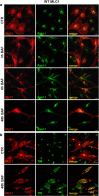
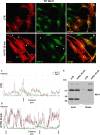
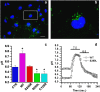
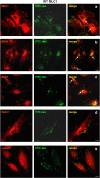
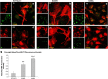
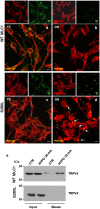
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is a rare leukodystrophy caused by mutations in the gene encoding MLC1, a membrane protein mainly expressed in astrocytes in the central nervous system. Although MLC1 function is unknown, evidence is emerging that it may regulate ion fluxes. Using biochemical and proteomic approaches to identify MLC1 interactors and elucidate MLC1 function we found that MLC1 interacts with the vacuolar ATPase (V-ATPase), the proton pump that regulates endosomal acidity. Because we previously showed that in intracellular organelles MLC1 directly binds Na, K-ATPase, which controls endosomal pH, we studied MLC1 endosomal localization and trafficking and MLC1 effects on endosomal acidity and function using human astrocytoma cells overexpressing wild-type (WT) MLC1 or MLC1 carrying pathological mutations. We found that WT MLC1 is abundantly expressed in early (EEA1(+), Rab5(+)) and recycling (Rab11(+)) endosomes and uses the latter compartment to traffic to the plasma membrane during hyposmotic stress. We also showed that WT MLC1 limits early endosomal acidification and influences protein trafficking in astrocytoma cells by stimulating protein recycling, as revealed by FITC-dextran measurement of endosomal pH and transferrin protein recycling assay, respectively. WT MLC1 also favors recycling to the plasma-membrane of the TRPV4 cation channel which cooperates with MLC1 to activate calcium influx in astrocytes during hyposmotic stress. Although MLC disease-causing mutations differentially affect MLC1 localization and trafficking, all the mutated proteins fail to influence endosomal pH and protein recycling. This study demonstrates that MLC1 modulates endosomal pH and protein trafficking suggesting that alteration of these processes contributes to MLC pathogenesis.
Keywords: Calcium; Early/recycling endosomes; Hyposmosis; K-ATPase; Leukodystrophy; Na; Rab11; TRPV4; V-ATPase.
Copyright © 2014. Published by Elsevier Inc.
Figures
Similar articles
-
Megalencephalic Leukoencephalopathy with Subcortical Cysts Disease-Linked MLC1 Protein Favors Gap-Junction Intercellular Communication by Regulating Connexin 43 Trafficking in Astrocytes.Cells. 2020 Jun 8;9(6):1425. doi: 10.3390/cells9061425. Cells. 2020. PMID: 32521795 Free PMC article.
-
Megalencephalic leukoencephalopathy with subcortical cysts protein 1 functionally cooperates with the TRPV4 cation channel to activate the response of astrocytes to osmotic stress: dysregulation by pathological mutations.Hum Mol Genet. 2012 May 15;21(10):2166-80. doi: 10.1093/hmg/dds032. Epub 2012 Feb 9. Hum Mol Genet. 2012. PMID: 22328087
-
Monocytes and macrophages as biomarkers for the diagnosis of megalencephalic leukoencephalopathy with subcortical cysts.Mol Cell Neurosci. 2013 Sep;56:307-21. doi: 10.1016/j.mcn.2013.07.001. Epub 2013 Jul 10. Mol Cell Neurosci. 2013. PMID: 23851226
-
Megalencephalic leukoencephalopathy with subcortical cysts: A personal biochemical retrospective.Eur J Med Genet. 2018 Jan;61(1):50-60. doi: 10.1016/j.ejmg.2017.10.013. Epub 2017 Oct 25. Eur J Med Genet. 2018. PMID: 29079544 Review.
-
GPR37 Receptors and Megalencephalic Leukoencephalopathy with Subcortical Cysts.Int J Mol Sci. 2022 May 16;23(10):5528. doi: 10.3390/ijms23105528. Int J Mol Sci. 2022. PMID: 35628339 Free PMC article. Review.
Cited by
-
Cerebellar Astrocyte Transduction as Gene Therapy for Megalencephalic Leukoencephalopathy.Neurotherapeutics. 2020 Oct;17(4):2041-2053. doi: 10.1007/s13311-020-00865-y. Neurotherapeutics. 2020. PMID: 32372403 Free PMC article.
-
Principles of Astrogliopathology.Adv Neurobiol. 2021;26:55-73. doi: 10.1007/978-3-030-77375-5_3. Adv Neurobiol. 2021. PMID: 34888830 Free PMC article.
-
Megalencephalic Leukoencephalopathy with Subcortical Cysts Disease-Linked MLC1 Protein Favors Gap-Junction Intercellular Communication by Regulating Connexin 43 Trafficking in Astrocytes.Cells. 2020 Jun 8;9(6):1425. doi: 10.3390/cells9061425. Cells. 2020. PMID: 32521795 Free PMC article.
-
Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy.Sci Rep. 2016 Sep 28;6:34325. doi: 10.1038/srep34325. Sci Rep. 2016. PMID: 27677466 Free PMC article.
-
Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research.Front Cell Neurosci. 2016 Sep 26;10:215. doi: 10.3389/fncel.2016.00215. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27725795 Free PMC article. Review.
References
-
- Agresti C., Aloisi F., Levi G. Heterotypic and homotypic cellular interactions influencing the growth and differentiation of bipotential oligodendrocyte-type-2 astrocyte progenitors in culture. Dev. Biol. 1991;144:16–29. - PubMed
-
- Ambrosini E., Serafini B., Lanciotti A., Tosini F., Scialpi F., Psaila R., Raggi C., Di Girolamo F., Petrucci T.C., Aloisi F. Biochemical characterization of MLC1 protein in astrocytes and its association with the dystrophin–glycoprotein complex. Mol. Cell. Neurosci. 2008;37:480–493. - PubMed
-
- Aoki T., Hagiwara H., Matsuzaki T., Suzuki T., Takata K. Internalization of caveolae and their relationship with endosomes in cultured human and mouse endothelial cells. Anat. Sci. Int. 2007;82:82–97. - PubMed
-
- Bacac M., Fusco C., Planche A., Santodomingo J., Demaurex N., Leemann-Zakaryan R., Provero P., Stamenkovic I. Securin and separase modulate membrane traffic by affecting endosomal acidification. Traffic. 2011;12:615–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

