A key regulatory role for Vav1 in controlling lipopolysaccharide endotoxemia via macrophage-derived IL-6
- PMID: 24532586
- PMCID: PMC3951479
- DOI: 10.4049/jimmunol.1300157
A key regulatory role for Vav1 in controlling lipopolysaccharide endotoxemia via macrophage-derived IL-6
Abstract
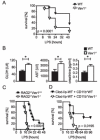
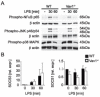
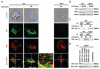
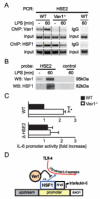
Macrophages are centrally involved in the pathogenesis of acute inflammatory diseases, peritonitis, endotoxemia, and septic shock. However, the molecular mechanisms controlling such macrophage activation are incompletely understood. In this article, we provide evidence that Vav1, a member of the RhoGEF family, plays a crucial role in macrophage activation and septic endotoxemia. Vav1-deficient mice demonstrated a significantly increased susceptibility for LPS endotoxemia that could be abrogated by anti-IL-6R Ab treatment. Subsequent studies showed that Vav1-deficient macrophages display augmented production of the proinflammatory cytokine IL-6. Nuclear Vav1 was identified as a key negative regulator of macrophage-derived IL-6 production. In fact, Vav1 formed a nuclear DNA-binding complex with heat shock transcription factor 1 at the HSE2 region of the IL-6 promoter to suppress IL-6 gene transcription in macrophages. These findings provide new insights into the pathogenesis of endotoxemia and suggest new avenues for therapy.
Figures

Similar articles
-
Reciprocal regulation of IL-6 and IL-10 balance by HGF via recruitment of heme oxygenase-1 in macrophages for attenuation of liver injury in a mouse model of endotoxemia.Int J Mol Med. 2009 Aug;24(2):161-70. doi: 10.3892/ijmm_00000219. Int J Mol Med. 2009. PMID: 19578789
-
Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation.J Immunol. 2002 Jun 15;168(12):6404-11. doi: 10.4049/jimmunol.168.12.6404. J Immunol. 2002. PMID: 12055259
-
Mitogen-activated protein kinase phosphatase-1 expression in macrophages is controlled by lymphocytes during macrophage activation.Int J Mol Med. 2012 Jan;29(1):25-31. doi: 10.3892/ijmm.2011.799. Epub 2011 Sep 28. Int J Mol Med. 2012. PMID: 21956667
-
Role of vav1 in the lipopolysaccharide-mediated upregulation of inducible nitric oxide synthase production and nuclear factor for interleukin-6 expression activity in murine macrophages.Clin Diagn Lab Immunol. 2004 May;11(3):525-31. doi: 10.1128/CDLI.11.3.525-531.2004. Clin Diagn Lab Immunol. 2004. PMID: 15138177 Free PMC article.
-
Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia.PLoS Pathog. 2012;8(5):e1002717. doi: 10.1371/journal.ppat.1002717. Epub 2012 May 24. PLoS Pathog. 2012. PMID: 22654663 Free PMC article.
Cited by
-
Development and Characterization of an Endotoxemia Model in Zebra Fish.Front Immunol. 2018 Mar 29;9:607. doi: 10.3389/fimmu.2018.00607. eCollection 2018. Front Immunol. 2018. PMID: 29651289 Free PMC article.
-
Adult astrocytes from reptiles are resistant to proinflammatory activation via sustaining Vav1 expression.J Biol Chem. 2021 Jan-Jun;296:100527. doi: 10.1016/j.jbc.2021.100527. Epub 2021 Mar 9. J Biol Chem. 2021. PMID: 33705794 Free PMC article.
-
Endotoxemia shifts neutrophils with TIMP-free gelatinase B/MMP-9 from bone marrow to the periphery and induces systematic upregulation of TIMP-1.Haematologica. 2017 Oct;102(10):1671-1682. doi: 10.3324/haematol.2017.168799. Epub 2017 Aug 3. Haematologica. 2017. PMID: 28775117 Free PMC article.
-
Effect of bone marrow mesenchymal stem cells on the polarization of macrophages.Mol Med Rep. 2018 Mar;17(3):4449-4459. doi: 10.3892/mmr.2018.8457. Epub 2018 Jan 18. Mol Med Rep. 2018. PMID: 29363724 Free PMC article.
-
Vav family exchange factors: an integrated regulatory and functional view.Small GTPases. 2014;5(2):9. doi: 10.4161/21541248.2014.973757. Small GTPases. 2014. PMID: 25483299 Free PMC article. Review.
References
-
- Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5:123–132. - PubMed
-
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. - PubMed
-
- Gosemann JH, van Griensven M, Barkhausen T, Kobbe P, Thobe BM, Haasper C, Pape HC, Krettek C, Hildebrand F, Frink M. TLR4 influences the humoral and cellular immune response during polymicrobial sepsis. Injury. 2010;41:1060–1067. - PubMed
-
- Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

