Altered macrophage phenotype transition impairs skeletal muscle regeneration
- PMID: 24525152
- PMCID: PMC3969996
- DOI: 10.1016/j.ajpath.2013.12.020
Altered macrophage phenotype transition impairs skeletal muscle regeneration
Abstract
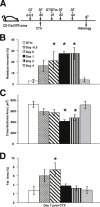
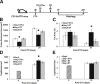
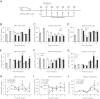
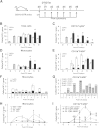
Monocyte/macrophage polarization in skeletal muscle regeneration is ill defined. We used CD11b-diphtheria toxin receptor transgenic mice to transiently deplete monocytes/macrophages at multiple stages before and after muscle injury induced by cardiotoxin. Fat accumulation within regenerated muscle was maximal when ablation occurred at the same time as cardiotoxin-induced injury. Early ablation (day 1 after cardiotoxin) resulted in the smallest regenerated myofiber size together with increased residual necrotic myofibers and fat accumulation. However, muscle regeneration after late (day 4) ablation was similar to controls. Levels of inflammatory cells in injured muscle following early ablation and associated with impaired muscle regeneration were determined by flow cytometry. Delayed, but exaggerated, monocyte [CD11b(+)(CD90/B220/CD49b/NK1.1/Ly6G)(-)(F4/80/I-Ab/CD11c)(-)Ly6C(+/-)] accumulation occurred; interestingly, Ly6C(+) and Ly6C(-) monocytes were present concurrently in ablated animals and control mice. In addition to monocytes, proinflammatory, Ly6C(+) macrophage accumulation following early ablation was delayed compared to controls. In both groups, CD11b(+)F4/80(+) cells exhibited minimal expression of the M2 markers CD206 and CD301. Nevertheless, early ablation delayed and decreased the transient accumulation of CD11b(+)F4/80(+)Ly6C(-)CD301(-) macrophages; in control animals, the later tissue accumulation of these cells appeared to correspond to that of anti-inflammatory macrophages, determined by cytokine production and arginase activity. In summary, impairments in muscle regeneration were associated with exaggerated monocyte recruitment and reduced Ly6C(-) macrophages; the switch of macrophage/monocyte subsets is critical to muscle regeneration.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Endogenous Uteroglobin as Intrinsic Anti-inflammatory Signal Modulates Monocyte and Macrophage Subsets Distribution Upon Sepsis Induced Lung Injury.Front Immunol. 2019 Oct 1;10:2276. doi: 10.3389/fimmu.2019.02276. eCollection 2019. Front Immunol. 2019. PMID: 31632392 Free PMC article.
-
Ly6CHi Blood Monocyte/Macrophage Drive Chronic Inflammation and Impair Wound Healing in Diabetes Mellitus.Arterioscler Thromb Vasc Biol. 2018 May;38(5):1102-1114. doi: 10.1161/ATVBAHA.118.310703. Epub 2018 Mar 1. Arterioscler Thromb Vasc Biol. 2018. PMID: 29496661 Free PMC article.
-
Absence of CCR2 results in an inflammaging environment in young mice with age-independent impairments in muscle regeneration.J Leukoc Biol. 2016 Nov;100(5):1011-1025. doi: 10.1189/jlb.3MA0316-104R. Epub 2016 Aug 16. J Leukoc Biol. 2016. PMID: 27531927 Free PMC article.
-
Macrophage plasticity in skeletal muscle repair.Biomed Res Int. 2014;2014:560629. doi: 10.1155/2014/560629. Epub 2014 Apr 17. Biomed Res Int. 2014. PMID: 24860823 Free PMC article. Review.
-
The Role of Metabolic Remodeling in Macrophage Polarization and Its Effect on Skeletal Muscle Regeneration.Antioxid Redox Signal. 2019 Apr 20;30(12):1553-1598. doi: 10.1089/ars.2017.7420. Epub 2018 Oct 9. Antioxid Redox Signal. 2019. PMID: 30070144 Review.
Cited by
-
Age-Associated Differences in Recovery from Exercise-Induced Muscle Damage.Cells. 2024 Jan 30;13(3):255. doi: 10.3390/cells13030255. Cells. 2024. PMID: 38334647 Free PMC article. Review.
-
Effect of limb demand ischemia on autophagy and morphology in mice.J Surg Res. 2015 Oct;198(2):515-24. doi: 10.1016/j.jss.2015.04.008. Epub 2015 Apr 9. J Surg Res. 2015. PMID: 25959834 Free PMC article.
-
Macrophages fine tune satellite cell fate in dystrophic skeletal muscle of mdx mice.PLoS Genet. 2019 Oct 18;15(10):e1008408. doi: 10.1371/journal.pgen.1008408. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31626629 Free PMC article.
-
Distinct Role of CD11b+Ly6G-Ly6C- Myeloid-Derived Cells on the Progression of the Primary Tumor and Therapy-Associated Recurrent Brain Tumor.Cells. 2019 Dec 24;9(1):51. doi: 10.3390/cells9010051. Cells. 2019. PMID: 31878276 Free PMC article.
-
Overexpression of Mechano-Growth Factor Modulates Inflammatory Cytokine Expression and Macrophage Resolution in Skeletal Muscle Injury.Front Physiol. 2018 Jul 26;9:999. doi: 10.3389/fphys.2018.00999. eCollection 2018. Front Physiol. 2018. PMID: 30140235 Free PMC article.
References
-
- Summan M., Warren G.L., Mercer R.R., Chapman R., Hulderman T., Van Rooijen N., Simeonova P.P. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. - PubMed
-
- Lin S.L., Castano A.P., Nowlin B.T., Lupher M.L., Jr., Duffield J.S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. - PubMed
-
- Geissmann F., Jung S., Littman D. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. - PubMed
-
- Swirski F., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L., Kohler R., Chudnovskiy A., Waterman P., Aikawa E., Mempel T., Libby P., Weissleder R., Pittet M. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

