MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses
- PMID: 24523886
- PMCID: PMC3921149
- DOI: 10.1371/journal.pone.0088340
MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses
Abstract
Background: The availability of a universal influenza vaccine able to induce broad cross-reactive immune responses against diverse influenza viruses would provide an alternative to currently available strain-specific vaccines. We evaluated the ability of vectors based on modified vaccinia virus Ankara (MVA) expressing conserved influenza proteins to protect mice against lethal challenge with multiple influenza subtypes.
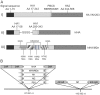
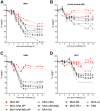
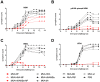
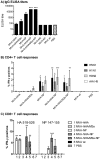
Methods: Mice were immunized with MVA vectors expressing H5N1-derived nucleoprotein (NP), the stem region of hemagglutinin (HA), matrix proteins 1 and 2 (M1 and M2), the viral polymerase basic protein 1 (PB1), or the HA stem fused to a quadrivalent matrix protein 2 extracellular domain (M2e). Immunized mice were challenged with lethal doses of H5N1, H7N1 or H9N2 virus and monitored for disease symptoms and weight loss. To investigate the influence of previous exposure to influenza virus on protective immune responses induced by conserved influenza proteins, mice were infected with pandemic H1N1 virus (H1N1pdm09) prior to immunization and subsequently challenged with H5N1 virus. Antibody and T cell responses were assessed by ELISA and flow cytometry, respectively.
Results: MVA vectors expressing NP alone, or co-expressed with other conserved influenza proteins, protected mice against lethal challenge with H5N1, H7N1 or H9N2 virus. Pre-exposure to H1N1pdm09 increased protective efficacy against lethal H5N1 challenge. None of the other conserved influenza proteins provided significant levels of protection against lethal challenge. NP-expressing vectors induced high numbers of influenza-specific CD4(+) and CD8(+) T cells and high titer influenza-specific antibody responses. Higher influenza-specific CD4(+) T cell responses and NP-specific CD8(+) T cell responses were associated with increased protective efficacy.
Conclusions: MVA vectors expressing influenza NP protect mice against lethal challenge with H5N1, H7N1 and H9N2 viruses by a mechanism involving influenza-specific CD4(+) and CD8(+) T cell responses.
Conflict of interest statement
Figures
Similar articles
-
Influenza Antigens NP and M2 Confer Cross Protection to BALB/c Mice against Lethal Challenge with H1N1, Pandemic H1N1 or H5N1 Influenza A Viruses.Viruses. 2021 Aug 27;13(9):1708. doi: 10.3390/v13091708. Viruses. 2021. PMID: 34578289 Free PMC article.
-
Mosaic H5 Hemagglutinin Provides Broad Humoral and Cellular Immune Responses against Influenza Viruses.J Virol. 2016 Jul 11;90(15):6771-6783. doi: 10.1128/JVI.00730-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27194759 Free PMC article.
-
Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques.J Virol. 2014 Nov;88(22):13418-28. doi: 10.1128/JVI.01219-14. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210172 Free PMC article.
-
Candidate influenza vaccines based on recombinant modified vaccinia virus Ankara.Expert Rev Vaccines. 2009 Apr;8(4):447-54. doi: 10.1586/erv.09.4. Expert Rev Vaccines. 2009. PMID: 19348560 Free PMC article. Review.
-
Towards a universal influenza vaccine: volunteer virus challenge studies in quarantine to speed the development and subsequent licensing.Br J Clin Pharmacol. 2013 Aug;76(2):210-6. doi: 10.1111/bcp.12146. Br J Clin Pharmacol. 2013. PMID: 23617282 Free PMC article. Review.
Cited by
-
Vaccination potential of B and T epitope-enriched NP and M2 against Influenza A viruses from different clades and hosts.PLoS One. 2018 Jan 29;13(1):e0191574. doi: 10.1371/journal.pone.0191574. eCollection 2018. PLoS One. 2018. PMID: 29377916 Free PMC article.
-
Modified vaccinia virus ankara (MVA) as production platform for vaccines against influenza and other viral respiratory diseases.Viruses. 2014 Jul 17;6(7):2735-61. doi: 10.3390/v6072735. Viruses. 2014. PMID: 25036462 Free PMC article. Review.
-
Immunogenicity and Protection Against Influenza H7N3 in Mice by Modified Vaccinia Virus Ankara Vectors Expressing Influenza Virus Hemagglutinin or Neuraminidase.Sci Rep. 2018 Mar 29;8(1):5364. doi: 10.1038/s41598-018-23712-9. Sci Rep. 2018. PMID: 29599502 Free PMC article.
-
M2e-Based Universal Influenza A Vaccines.Vaccines (Basel). 2015 Feb 13;3(1):105-36. doi: 10.3390/vaccines3010105. Vaccines (Basel). 2015. PMID: 26344949 Free PMC article. Review.
-
The Next Generation of Influenza Vaccines: Towards a Universal Solution.Vaccines (Basel). 2021 Jan 7;9(1):26. doi: 10.3390/vaccines9010026. Vaccines (Basel). 2021. PMID: 33430278 Free PMC article. Review.
References
-
- Watanabe Y, Ibrahim MS, Suzuki Y, Ikuta K (2012) The changing nature of avian influenza A virus (H5N1). Trends Microbiol 20: 11–20. - PubMed
-
- Cox NJ, Subbarao K (1999) Influenza. Lancet 354: 1277–1282. - PubMed
-
- Donis RO, Cox NJ (2011) Prospecting the influenza hemagglutinin to develop universal vaccines. Clin Infect Dis 52: 1010–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

