Levels of CD56+TIM-3- effector CD8 T cells distinguish HIV natural virus suppressors from patients receiving antiretroviral therapy
- PMID: 24520422
- PMCID: PMC3919829
- DOI: 10.1371/journal.pone.0088884
Levels of CD56+TIM-3- effector CD8 T cells distinguish HIV natural virus suppressors from patients receiving antiretroviral therapy
Abstract
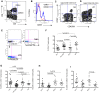
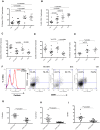
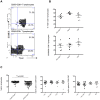
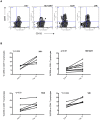
Prolonged antiretroviral therapy (ART) with effective HIV suppression and reconstitution of CD4 T cells, fails to restore CD8 T cell lytic effector function that is needed to eradicate the viral reservoir. Better understanding of the phenotype and function of circulating CD8 cells in HIV patients will contribute to new targeted therapies directed at increasing CD8 T cell lytic effector function and destruction of the viral reservoir. We show that CD8 T cells from ART treated patients had sharply reduced expression of CD56 (neural cell adhesion molecule-1), a marker associated with cytolytic function whereas elite patients who control HIV in the absence of ART had CD56+ CD8 T cell levels similar to uninfected controls. The CD56+ CD8 T cells had higher perforin upregulation as well as degranulation following stimulation with HIV gag peptides compared with CD56 negative CD8 T cells. Elite patients had the highest frequencies of perforin producing CD56+ CD8 T cells among all HIV+ groups. In patients receiving ART we noted high levels of the exhaustion marker TIM-3 on CD56+ CD8 T cells, implying that defective effector function was related to immune exhaustion. CD56+ CD8 T cells from elite or treated HIV patients responded to PMA plus ionomycin stimulation, and expressed transcription factors T-bet and EOMES at levels similar to uninfected controls. Consequently, the lytic effector defect in chronic HIV disease is due to immune exhaustion and quantitative loss of CD56+ CD8 T cells and this defect is not repaired in patients where viremia is suppressed and CD4 T cells are recovered after ART. Reconstituting the cytotoxic CD56+ subset of CD8+ T cells through new interventions might improve the lytic effector capacity and contribute to reducing the viral reservoir. Our initial studies indicate that IL-15 treatment partly reverses the CD56 defect, implying that myeloid cell defects could be targeted for immune therapy during chronic HIV disease.
Conflict of interest statement
Figures

Similar articles
-
Tim-3 negatively regulates cytotoxicity in exhausted CD8+ T cells in HIV infection.PLoS One. 2012;7(7):e40146. doi: 10.1371/journal.pone.0040146. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792231 Free PMC article.
-
Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression.Blood. 2011 Apr 7;117(14):3799-808. doi: 10.1182/blood-2010-12-322727. Epub 2011 Feb 2. Blood. 2011. PMID: 21289310 Free PMC article.
-
Granule-dependent mechanisms of lysis are defective in CD8 T cells of HIV-infected, antiretroviral therapy-treated individuals.AIDS. 2004 Apr 9;18(6):859-69. doi: 10.1097/00002030-200404090-00003. AIDS. 2004. PMID: 15060433
-
Harnessing Autophagy to Overcome Antigen-Specific T-Cell Dysfunction: Implication for People Living with HIV-1.Int J Mol Sci. 2023 Jul 3;24(13):11018. doi: 10.3390/ijms241311018. Int J Mol Sci. 2023. PMID: 37446195 Free PMC article. Review.
-
Enhancing Human Immunodeficiency Virus-Specific CD8(+) T Cell Responses with Heteroclitic Peptides.Front Immunol. 2015 Jul 23;6:377. doi: 10.3389/fimmu.2015.00377. eCollection 2015. Front Immunol. 2015. PMID: 26257743 Free PMC article. Review.
Cited by
-
Synthetic consensus HIV-1 DNA induces potent cellular immune responses and synthesis of granzyme B, perforin in HIV infected individuals.Mol Ther. 2015 Mar;23(3):591-601. doi: 10.1038/mt.2014.245. Epub 2014 Dec 22. Mol Ther. 2015. PMID: 25531694 Free PMC article.
-
CD56 in the Immune System: More Than a Marker for Cytotoxicity?Front Immunol. 2017 Jul 24;8:892. doi: 10.3389/fimmu.2017.00892. eCollection 2017. Front Immunol. 2017. PMID: 28791027 Free PMC article. Review.
-
Natural Killer T-like Cells: Immunobiology and Role in Disease.Int J Mol Sci. 2023 Feb 1;24(3):2743. doi: 10.3390/ijms24032743. Int J Mol Sci. 2023. PMID: 36769064 Free PMC article. Review.
-
TIM-3 rs1036199 polymorphism increases susceptibility to autoimmune diseases: evidence based on 4200 subjects.Biosci Rep. 2018 Nov 23;38(6):BSR20181235. doi: 10.1042/BSR20181235. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30377229 Free PMC article.
-
The Roles of Coinhibitory Receptors in Pathogenesis of Human Retroviral Infections.Front Immunol. 2018 Nov 27;9:2755. doi: 10.3389/fimmu.2018.02755. eCollection 2018. Front Immunol. 2018. PMID: 30538707 Free PMC article. Review.
References
-
- Trabattoni D, Piconi S, Biasin M, Rizzardini G, Migliorino M, et al. (2004) Granule-dependent mechanisms of lysis are defective in CD8 T cells of HIV-infected, antiretroviral therapy-treated individuals. AIDS 18: 859–869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

