Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells
- PMID: 24520093
- PMCID: PMC4557204
- DOI: 10.1158/1078-0432.CCR-13-2817
Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells
Abstract
Purpose: Myeloma-directed cellular immune responses after autologous stem cell transplantation (ASCT) may reduce relapse rates. We studied whether coinjecting the TLR-3 agonist and vaccine adjuvant Poly-ICLC with a MAGE-A3 peptide vaccine was safe and would elicit a high frequency of vaccine-directed immune responses when combined with vaccine-primed and costimulated autologous T cells.
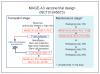
Experimental design: In a phase II clinical trial (NCT01245673), we evaluated the safety and activity of ex vivo expanded autologous T cells primed in vivo using a MAGE-A3 multipeptide vaccine (compound GL-0817) combined with Poly-ICLC (Hiltonol), granulocyte macrophage colony-stimulating factor (GM-CSF) ± montanide. Twenty-seven patients with active and/or high-risk myeloma received autografts followed by anti-CD3/anti-CD28-costimulated autologous T cells, accompanied by MAGE-A3 peptide immunizations before T-cell collection and five times after ASCT. Immune responses to the vaccine were evaluated by cytokine production (all patients), dextramer binding to CD8(+) T cells, and ELISA performed serially after transplant.
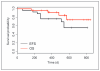
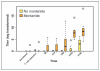
Results: T-cell infusions were well tolerated, whereas vaccine injection site reactions occurred in >90% of patients. Two of nine patients who received montanide developed sterile abscesses; however, this did not occur in the 18 patients who did not receive montanide. Dextramer staining demonstrated MAGE-A3-specific CD8 T cells in 7 of 8 evaluable HLA-A2(+) patients (88%), whereas vaccine-specific cytokine-producing T cells were generated in 19 of 25 patients (76%). Antibody responses developed in 7 of 9 patients (78%) who received montanide and only weakly in 2 of 18 patients (11%) who did not. The 2-year overall survival was 74% [95% confidence interval (CI), 54%-100%] and 2-year event-free survival was 56% (95% CI, 37%-85%).
Conclusions: A high frequency of vaccine-specific T-cell responses were generated after transplant by combining costimulated autologous T cells with a Poly-ICLC/GM-CSF-primed MAGE-A3 vaccine.
©2013 AACR
Figures

Similar articles
-
Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients.Clin Cancer Res. 2012 Dec 1;18(23):6497-508. doi: 10.1158/1078-0432.CCR-12-2189. Epub 2012 Oct 2. Clin Cancer Res. 2012. PMID: 23032745 Clinical Trial.
-
Combined Vaccination with NY-ESO-1 Protein, Poly-ICLC, and Montanide Improves Humoral and Cellular Immune Responses in Patients with High-Risk Melanoma.Cancer Immunol Res. 2020 Jan;8(1):70-80. doi: 10.1158/2326-6066.CIR-19-0545. Epub 2019 Nov 7. Cancer Immunol Res. 2020. PMID: 31699709 Free PMC article. Clinical Trial.
-
Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma.Blood. 2011 Jan 20;117(3):788-97. doi: 10.1182/blood-2010-08-299396. Epub 2010 Oct 28. Blood. 2011. PMID: 21030558 Free PMC article. Clinical Trial.
-
Poly-ICLC, a multi-functional immune modulator for treating cancer.Semin Immunol. 2020 Jun;49:101414. doi: 10.1016/j.smim.2020.101414. Epub 2020 Oct 1. Semin Immunol. 2020. PMID: 33011064 Review.
-
A systematic review on poly(I:C) and poly-ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy.J Exp Clin Cancer Res. 2021 Jun 25;40(1):213. doi: 10.1186/s13046-021-02017-2. J Exp Clin Cancer Res. 2021. PMID: 34172082 Free PMC article. Review.
Cited by
-
Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses.Nat Med. 2016 Dec;22(12):1402-1410. doi: 10.1038/nm.4200. Epub 2016 Oct 24. Nat Med. 2016. PMID: 27775706 Free PMC article.
-
Adoptive Cellular Therapy for Multiple Myeloma Using CAR- and TCR-Transgenic T Cells: Response and Resistance.Cells. 2022 Jan 25;11(3):410. doi: 10.3390/cells11030410. Cells. 2022. PMID: 35159220 Free PMC article. Review.
-
In Silico Model Estimates the Clinical Trial Outcome of Cancer Vaccines.Cells. 2021 Nov 5;10(11):3048. doi: 10.3390/cells10113048. Cells. 2021. PMID: 34831269 Free PMC article.
-
Deregulation of Adaptive T Cell Immunity in Multiple Myeloma: Insights Into Mechanisms and Therapeutic Opportunities.Front Oncol. 2020 May 5;10:636. doi: 10.3389/fonc.2020.00636. eCollection 2020. Front Oncol. 2020. PMID: 32432039 Free PMC article. Review.
-
WT1 peptide vaccine in Montanide in contrast to poly ICLC, is able to induce WT1-specific immune response with TCR clonal enrichment in myeloid leukemia.Exp Hematol Oncol. 2018 Jan 11;7:1. doi: 10.1186/s40164-018-0093-x. eCollection 2018. Exp Hematol Oncol. 2018. PMID: 29344432 Free PMC article.
References
-
- Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus myeloma effect: proof of principle. Blood. 1996;87:1196–8. - PubMed
-
- Barlogie B, Jagannath S, Vesole DH, Naucke S, Cheson B, Mattox S, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–93. - PubMed
-
- Porrata LF, Gertz MA, Inwards DJ, Utzow MR, Lacy MQ, Tefferi A, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin Iymphoma. Blood. 2001;98:579–85. - PubMed
-
- Porrata LF, Markovic SN. Timely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantation. Clin Exp Med. 2004;4:78–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

