Aromatic-aromatic interactions enhance interfiber contacts for enzymatic formation of a spontaneously aligned supramolecular hydrogel
- PMID: 24512553
- PMCID: PMC3986011
- DOI: 10.1021/ja4127399
Aromatic-aromatic interactions enhance interfiber contacts for enzymatic formation of a spontaneously aligned supramolecular hydrogel
Abstract
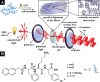
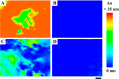
Anisotropy or alignment is a critical feature of functional soft materials in living organisms, but it remains a challenge for spontaneously generating anisotropic gel materials. Here we report a molecular design that increases intermolecular aromatic-aromatic interactions of hydrogelators during enzymatic hydrogelation for spontaneously forming an anisotropic hydrogel. This process, relying on both aromatic-aromatic interactions and enzyme catalysis, results in spontaneously aligned supramolecular nanofibers as the matrices of a monodomain hydrogel that exhibits significant birefringence. This work, as the first example of monodomain hydrogels formed via an enzymatic reaction, illustrates a new biomimetic approach for generating aligned anisotropic soft materials.
Figures



Similar articles
-
Enzymatic Dissolution of Biocomposite Solids Consisting of Phosphopeptides to Form Supramolecular Hydrogels.Chemistry. 2015 Dec 7;21(50):18047-51. doi: 10.1002/chem.201504087. Epub 2015 Oct 29. Chemistry. 2015. PMID: 26462722 Free PMC article.
-
Covalent-supramolecular hybrid polymers as muscle-inspired anisotropic actuators.Nat Commun. 2018 Jun 19;9(1):2395. doi: 10.1038/s41467-018-04800-w. Nat Commun. 2018. PMID: 29921928 Free PMC article.
-
Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels.J Am Chem Soc. 2010 Mar 3;132(8):2719-28. doi: 10.1021/ja9088764. J Am Chem Soc. 2010. PMID: 20131781
-
Enzyme-manipulated hydrogelation of small molecules for biomedical applications.Acta Biomater. 2022 Oct 1;151:88-105. doi: 10.1016/j.actbio.2022.08.016. Epub 2022 Aug 13. Acta Biomater. 2022. PMID: 35970483 Review.
-
Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches.Acc Chem Res. 2017 Apr 18;50(4):740-750. doi: 10.1021/acs.accounts.7b00070. Epub 2017 Mar 2. Acc Chem Res. 2017. PMID: 28252940 Review.
Cited by
-
Aromatic-Aromatic Interactions Enable α-Helix to β-Sheet Transition of Peptides to Form Supramolecular Hydrogels.J Am Chem Soc. 2017 Jan 11;139(1):71-74. doi: 10.1021/jacs.6b11512. Epub 2016 Dec 22. J Am Chem Soc. 2017. PMID: 27997165 Free PMC article.
-
Self-assembly of nucleopeptides to interact with DNAs.Interface Focus. 2017 Dec 6;7(6):20160116. doi: 10.1098/rsfs.2016.0116. Epub 2017 Oct 20. Interface Focus. 2017. PMID: 29147550 Free PMC article.
-
Ligand-Receptor Interaction Modulates the Energy Landscape of Enzyme-Instructed Self-Assembly of Small Molecules.J Am Chem Soc. 2016 Nov 30;138(47):15397-15404. doi: 10.1021/jacs.6b07677. Epub 2016 Nov 15. J Am Chem Soc. 2016. PMID: 27797504 Free PMC article.
-
Synthesis and bioactivity of pyrrole-conjugated phosphopeptides.Beilstein J Org Chem. 2022 Jan 31;18:159-166. doi: 10.3762/bjoc.18.17. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 35186152 Free PMC article.
-
Peptide Tectonics: Encoded Structural Complementarity Dictates Programmable Self-Assembly.Adv Sci (Weinh). 2019 Apr 29;6(13):1802043. doi: 10.1002/advs.201802043. eCollection 2019 Jul 3. Adv Sci (Weinh). 2019. PMID: 31380179 Free PMC article. Review.
References
-
- Gray G. W. Liq. Cryst. 1998, 24, 5.
-
- Cox J. R.; Simpson J. H.; Swager T. M. J. Am. Chem. Soc. 2013, 135, 640. - PubMed
- Hoogboom J.; Swager T. M. J. Am. Chem. Soc. 2006, 128, 15058. - PubMed
- Kato T.; Yasuda T.; Kamikawa Y.; Yoshio M. Chem. Commun. 2009, 729. - PubMed
- Kato T.; Mizoshita N.; Kanie K. Macromol. Rapid Commun. 2001, 22, 797.
-
- Zhang S. M.; Greenfield M. A.; Mata A.; Palmer L. C.; Bitton R.; Mantei J. R.; Aparicio C.; de la Cruz M. O.; Stupp S. I. Nat. Mater. 2010, 9, 594. - PMC - PubMed
- Wu Z. L.; Kurokawa T.; Liang S.; Furukawa H.; Gong J. P. J. Am. Chem. Soc. 2010, 132, 10064. - PubMed
- Zhao F.; Gao Y. A.; Shi J. F.; Browdy H. M.; Xu B. Langmuir 2011, 27, 1510. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

