TGF-β: duality of function between tumor prevention and carcinogenesis
- PMID: 24511106
- PMCID: PMC3952197
- DOI: 10.1093/jnci/djt369
TGF-β: duality of function between tumor prevention and carcinogenesis
Abstract
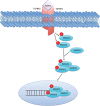
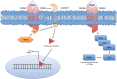
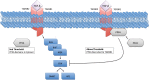
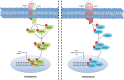
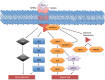
Several mechanisms underlying tumor progression have remained elusive, particularly in relation to transforming growth factor beta (TGF-β). Although TGF-β initially inhibits epithelial growth, it appears to promote the progression of advanced tumors. Defects in normal TGF-β pathways partially explain this paradox, which can lead to a cascade of downstream events that drive multiple oncogenic pathways, manifesting as several key features of tumorigenesis (uncontrolled proliferation, loss of apoptosis, epithelial-to-mesenchymal transition, sustained angiogenesis, evasion of immune surveillance, and metastasis). Understanding the mechanisms of TGF-β dysregulation will likely reveal novel points of convergence between TGF-β and other pathways that can be specifically targeted for therapy.
Figures
Similar articles
-
Lack of transforming growth factor-β signaling promotes collective cancer cell invasion through tumor-stromal crosstalk.Breast Cancer Res. 2012 Jul 2;14(4):R98. doi: 10.1186/bcr3217. Breast Cancer Res. 2012. PMID: 22748014 Free PMC article.
-
The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer.Oncogene. 2012 Dec 13;31(50):5162-71. doi: 10.1038/onc.2012.11. Epub 2012 Jan 30. Oncogene. 2012. PMID: 22286770 Free PMC article.
-
Proteinase-Activated Receptor 2 May Drive Cancer Progression by Facilitating TGF-β Signaling.Int J Mol Sci. 2017 Nov 22;18(11):2494. doi: 10.3390/ijms18112494. Int J Mol Sci. 2017. PMID: 29165389 Free PMC article. Review.
-
Vasohibin-2 is required for epithelial-mesenchymal transition of ovarian cancer cells by modulating transforming growth factor-β signaling.Cancer Sci. 2017 Mar;108(3):419-426. doi: 10.1111/cas.13157. Cancer Sci. 2017. PMID: 28064471 Free PMC article.
-
Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer.Biochim Biophys Acta. 2007 Jan;1775(1):21-62. doi: 10.1016/j.bbcan.2006.06.004. Epub 2006 Jul 8. Biochim Biophys Acta. 2007. PMID: 16904831 Review.
Cited by
-
Orphan nuclear receptor COUP-TFII enhances myofibroblast glycolysis leading to kidney fibrosis.EMBO Rep. 2021 Jun 4;22(6):e51169. doi: 10.15252/embr.202051169. Epub 2021 May 25. EMBO Rep. 2021. PMID: 34031962 Free PMC article.
-
The platinum coordination complex inhibits cell invasion-migration and epithelial-to-mesenchymal transition by altering the TGF-β-SMAD pathway in colorectal cancer.Front Pharmacol. 2023 Nov 8;14:1178190. doi: 10.3389/fphar.2023.1178190. eCollection 2023. Front Pharmacol. 2023. PMID: 38027033 Free PMC article.
-
TCR signaling intensity controls CD8+ T cell responsiveness to TGF-β.J Leukoc Biol. 2015 Nov;98(5):703-12. doi: 10.1189/jlb.2HIMA1214-578R. Epub 2015 Jul 7. J Leukoc Biol. 2015. PMID: 26153417 Free PMC article.
-
Targeted therapies in hepatocellular carcinoma: past, present, and future.Front Oncol. 2024 Aug 29;14:1432423. doi: 10.3389/fonc.2024.1432423. eCollection 2024. Front Oncol. 2024. PMID: 39267840 Free PMC article. Review.
-
Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Esophageal Adenocarcinoma: Results from a Phase 1 Cohort.Target Oncol. 2021 Jul;16(4):435-446. doi: 10.1007/s11523-021-00809-2. Epub 2021 May 19. Target Oncol. 2021. PMID: 34009501 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

