Necroptosis drives motor neuron death in models of both sporadic and familial ALS
- PMID: 24508385
- PMCID: PMC3951532
- DOI: 10.1016/j.neuron.2014.01.011
Necroptosis drives motor neuron death in models of both sporadic and familial ALS
Abstract
Most cases of neurodegenerative diseases are sporadic, hindering the use of genetic mouse models to analyze disease mechanisms. Focusing on the motor neuron (MN) disease amyotrophic lateral sclerosis (ALS), we therefore devised a fully humanized coculture model composed of human adult primary sporadic ALS (sALS) astrocytes and human embryonic stem-cell-derived MNs. The model reproduces the cardinal features of human ALS: sALS astrocytes, but not those from control patients, trigger selective death of MNs. The mechanisms underlying this non-cell-autonomous toxicity were investigated in both astrocytes and MNs. Although causal in familial ALS (fALS), SOD1 does not contribute to the toxicity of sALS astrocytes. Death of MNs triggered by either sALS or fALS astrocytes occurs through necroptosis, a form of programmed necrosis involving receptor-interacting protein 1 and the mixed lineage kinase domain-like protein. The necroptotic pathway therefore constitutes a potential therapeutic target for this incurable disease.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
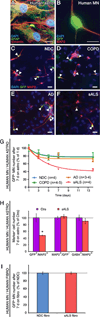
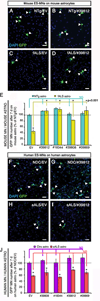
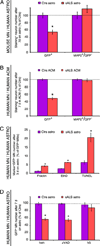
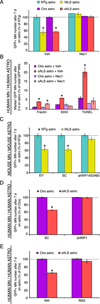
Comment in
-
Motor neuron death in ALS: programmed by astrocytes?Neuron. 2014 Mar 5;81(5):961-963. doi: 10.1016/j.neuron.2014.02.024. Neuron. 2014. PMID: 24607221 Free PMC article.
Similar articles
-
Astrocytes from familial and sporadic ALS patients are toxic to motor neurons.Nat Biotechnol. 2011 Aug 10;29(9):824-8. doi: 10.1038/nbt.1957. Nat Biotechnol. 2011. PMID: 21832997 Free PMC article.
-
Deletion of Ripk3 Prevents Motor Neuron Death In Vitro but not In Vivo.eNeuro. 2019 Feb 19;6(1):ENEURO.0308-18.2018. doi: 10.1523/ENEURO.0308-18.2018. eCollection 2019 Jan-Feb. eNeuro. 2019. PMID: 30815534 Free PMC article.
-
Motor neuron death in ALS: programmed by astrocytes?Neuron. 2014 Mar 5;81(5):961-963. doi: 10.1016/j.neuron.2014.02.024. Neuron. 2014. PMID: 24607221 Free PMC article.
-
Rodent Models of Amyotrophic Lateral Sclerosis.Curr Protoc Pharmacol. 2015 Jun 1;69:5.67.1-5.67.21. doi: 10.1002/0471141755.ph0567s69. Curr Protoc Pharmacol. 2015. PMID: 26344214 Free PMC article. Review.
-
Recent advances in research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 gene mutations: neuronal Lewy body-like hyaline inclusions and astrocytic hyaline inclusions.Histol Histopathol. 1999 Jul;14(3):973-89. doi: 10.14670/HH-14.973. Histol Histopathol. 1999. PMID: 10425565 Review.
Cited by
-
Post-translational modifications as key regulators of TNF-induced necroptosis.Cell Death Dis. 2016 Jul 7;7(7):e2293. doi: 10.1038/cddis.2016.197. Cell Death Dis. 2016. PMID: 27383048 Free PMC article. Review.
-
Roles of receptor-interacting protein kinase 1 in SH-SY5Y cells with beta amyloid-induced neurotoxicity.J Cell Mol Med. 2022 Mar;26(5):1434-1444. doi: 10.1111/jcmm.17095. Epub 2022 Feb 2. J Cell Mol Med. 2022. PMID: 35106914 Free PMC article.
-
Crashing the computer: apoptosis vs. necroptosis in neuroinflammation.Cell Death Differ. 2019 Jan;26(1):41-52. doi: 10.1038/s41418-018-0195-3. Epub 2018 Oct 19. Cell Death Differ. 2019. PMID: 30341422 Free PMC article. Review.
-
Impact of traumatic brain injury on amyotrophic lateral sclerosis: from bedside to bench.J Neurophysiol. 2019 Sep 1;122(3):1174-1185. doi: 10.1152/jn.00572.2018. Epub 2019 May 22. J Neurophysiol. 2019. PMID: 31116639 Free PMC article. Review.
-
The role of necroptosis in the treatment of diseases.BMB Rep. 2018 May;51(5):219-224. doi: 10.5483/bmbrep.2018.51.5.074. BMB Rep. 2018. PMID: 29636122 Free PMC article. Review.
References
-
- Bates DM, Watts DG. Nonlinear Regression Analysis and Its Applications. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 1988.
-
- Cassina P, Pehar M, Vargas MR, Castellanos R, Barbeito AG, Estevez AG, Thompson JA, Beckman JS, Barbeito L. Astrocyte activation by fibroblast growth factor-1 and motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J. Neurochem. 2005;93:38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS078614/NS/NINDS NIH HHS/United States
- NS072182-01/NS/NINDS NIH HHS/United States
- TL1 TR000082/TR/NCATS NIH HHS/United States
- R21 NS072182/NS/NINDS NIH HHS/United States
- NS062055-01A1/NS/NINDS NIH HHS/United States
- 5 U42 RR006042/RR/NCRR NIH HHS/United States
- R21 NS064191/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- U42 RR006042/RR/NCRR NIH HHS/United States
- R01 NS062055/NS/NINDS NIH HHS/United States
- P30 ES009089/ES/NIEHS NIH HHS/United States
- R01ES160348/ES/NIEHS NIH HHS/United States
- ES009089/ES/NIEHS NIH HHS/United States
- R21 NS062180/NS/NINDS NIH HHS/United States
- NS042269-05A2/NS/NINDS NIH HHS/United States
- NS078614-01A1/NS/NINDS NIH HHS/United States
- NS064191-01A1/NS/NINDS NIH HHS/United States
- NS062180/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

