Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study
- PMID: 24508103
- PMCID: PMC4382632
- DOI: 10.1016/S1470-2045(14)70012-9
Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study
Abstract
Background: In the BRIM-3 trial, vemurafenib was associated with risk reduction versus dacarbazine of both death and progression in patients with advanced BRAF(V600) mutation-positive melanoma. We present an extended follow-up analysis of the total population and in the BRAF(V600E) and BRAF(V600K) mutation subgroups.
Methods: Patients older than 18 years, with treatment-naive metastatic melanoma and whose tumour tissue was positive for BRAF(V600) mutations were eligible. Patients also had to have a life expectancy of at least 3 months, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate haematological, hepatic, and renal function. Patients were randomly assigned by interactive voice recognition system to receive either vemurafenib (960 mg orally twice daily) or dacarbazine (1000 mg/m(2) of body surface area intravenously every 3 weeks). Coprimary endpoints were overall survival and progression-free survival, analysed in the intention-to-treat population (n=675), with data censored at crossover. A sensitivity analysis was done. This trial is registered with ClinicalTrials.gov, NCT01006980.
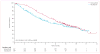
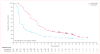
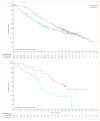
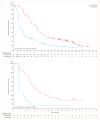
Findings: 675 eligible patients were enrolled from 104 centres in 12 countries between Jan 4, 2010, and Dec 16, 2010. 337 patients were randomly assigned to receive vemurafenib and 338 to receive dacarbazine. Median follow-up was 12·5 months (IQR 7·7-16·0) on vemurafenib and 9·5 months (3·1-14·7) on dacarbazine. 83 (25%) of the 338 patients initially randomly assigned to dacarbazine crossed over from dacarbazine to vemurafenib. Median overall survival was significantly longer in the vemurafenib group than in the dacarbazine group (13·6 months [95% CI 12·0-15·2] vs 9·7 months [7·9-12·8]; hazard ratio [HR] 0·70 [95% CI 0·57-0·87]; p=0·0008), as was median progression-free survival (6·9 months [95% CI 6·1-7·0] vs 1·6 months [1·6-2·1]; HR 0·38 [95% CI 0·32-0·46]; p<0·0001). For the 598 (91%) patients with BRAF(V600E) disease, median overall survival in the vemurafenib group was 13·3 months (95% CI 11·9-14·9) compared with 10·0 months (8·0-14·0) in the dacarbazine group (HR 0·75 [95% CI 0·60-0·93]; p=0·0085); median progression-free survival was 6·9 months (95% CI 6·2-7·0) and 1·6 months (1·6-2·1), respectively (HR 0·39 [95% CI 0·33-0·47]; p<0·0001). For the 57 (9%) patients with BRAF(V600K) disease, median overall survival in the vemurafenib group was 14·5 months (95% CI 11·2-not estimable) compared with 7·6 months (6·1-16·6) in the dacarbazine group (HR 0·43 [95% CI 0·21-0·90]; p=0·024); median progression-free survival was 5·9 months (95% CI 4·4-9·0) and 1·7 months (1·4-2·9), respectively (HR 0·30 [95% CI 0·16-0·56]; p<0·0001). The most frequent grade 3-4 events were cutaneous squamous-cell carcinoma (65 [19%] of 337 patients) and keratoacanthomas (34 [10%]), rash (30 [9%]), and abnormal liver function tests (38 [11%]) in the vemurafenib group and neutropenia (26 [9%] of 287 patients) in the dacarbazine group. Eight (2%) patients in the vemurafenib group and seven (2%) in the dacarbazine group had grade 5 events.
Interpretation: Inhibition of BRAF with vemurafenib improves survival in patients with the most common BRAF(V600E) mutation and in patients with the less common BRAF(V600K) mutation.
Funding: F Hoffmann-La Roche-Genentech.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
GAM has received research funding from Novartis, Pfizer, and Millennium, and has provided uncompensated services for Roche- Genentech, Plexxicon, Novartis, Bristol-Myers Squibb, GlaxoSmithKline, Amgen, and Millennium. PBC has received research support and served on advisory boards for GlaxoSmithKline, and has been a consultant for Bristol-Myers Squibb. CR has served as a consultant for Roche, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, and Merck. JL has served on advisory boards for Roche-Genentech, Bristol-Myers Squibb, and GlaxoSmithKline. JBH has served on advisory boards. AR has received honoraria for advisory boards from Roche-Genentech, paid to his institution. OH has been a speaker and consultant for Genentech and has received research funding. PAA has been a consultant or had an advisory role for Bristol-Myers Squibb, Merck Sharp & Dohme, Roche-Genentech, GlaxoSmithKline, Celgen, Amgen, and Novartis, and has received honoraria from Bristol-Myers Squibb, Roche-Genentech, and GlaxoSmithKline. AME has served on advisory boards for Bristol-Myers Squibb, GlaxoSmithKline, MedImuune, and Merck. AT has a consultant and advisory board relationship with and has received honoraria from Bristol-Myers Squibb, GlaxoSmithKline, Igea, Roche, and Amgen, and he has received honoraria from Oncovision. MM has received honoraria for advisory boards, consultancy, and speakers bureaus from Bristol-Myers Squibb, Roche-Genentech, and MedImmune, and research funds from Bristol-Myers Squibb. CL has been a consultant or has had an advisory board relationship with Roche, GlaxoSmithKline, Novartis, Bristol-Myers Squibb, and Amgen. TJ has been a consultant or had an advisory role and received research funding from Bristol-Myers Squibb, Roche-Genentech, and GlaxoSmithKline. DS has received research funding from Merck, and has had consultancies or participated in advisory boards with GlaxoSmithKline, Roche, Bristol Myers-Squibb, Merck, Amgen, Delcath, and Novartis. SJO’D receives research funding from GlaxoSmithKline. JMK has been a consultant or had an advisory board relationship with Bristol-Myers Squibb, Merck, Celgene, and Ziopharm. RD receives research funding from AstraZeneca, Novartis, Cephalon, Merck Sharp & Dohme, Transgene, Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Bayer, and has a consultant or advisory board relationship with AstraZeneca, Novartis, Cephalon, Merck Sharp & Dohme, Transgene, Genta, Bayer, Roche, Bristol-Myers Squibb, GlaxoSmithKline, and Spirig. BD has served on advisory boards for Roche-Genentech, Bristol-Myers Squibb, and GlaxoSmithKline. JAS has served on advisory boards for Genentech. KTF is a consultant for Roche-Genentech. MY is an employee of Roche-Genentech, with company stock. IC is an employee of Roche- Genentech, is a stockholder, and has stock options. SC is employed by Roche Molecular Systems Inc. KT is an employee of F Hoffman-LaRoche, with stock ownership. AH has received honoraria for advisory boards, consultancy, and speakers bureaus, as well as research funding from Amgen, Bristol-Myers Squibb, Celgene, Eisai, GlaxoSmithKline, MedImmune, MetaSciences, Merck Serono, MSD/Merck, Novartis, Oncosec, and Roche Pharma. The other authors declare that they have no conflicts of interest.
Figures
Comment in
-
Melanoma as a model for precision medicine in oncology.Lancet Oncol. 2014 Mar;15(3):251-3. doi: 10.1016/S1470-2045(14)70059-2. Epub 2014 Feb 7. Lancet Oncol. 2014. PMID: 24508105 No abstract available.
-
Melanoma: blocking BRAF to the BRIM.Nat Rev Clin Oncol. 2014 Apr;11(4):179. doi: 10.1038/nrclinonc.2014.32. Epub 2014 Feb 25. Nat Rev Clin Oncol. 2014. PMID: 24569445 No abstract available.
Similar articles
-
Improved survival with vemurafenib in melanoma with BRAF V600E mutation.N Engl J Med. 2011 Jun 30;364(26):2507-16. doi: 10.1056/NEJMoa1103782. Epub 2011 Jun 5. N Engl J Med. 2011. PMID: 21639808 Free PMC article. Clinical Trial.
-
Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2018 Oct;19(10):1315-1327. doi: 10.1016/S1470-2045(18)30497-2. Epub 2018 Sep 12. Lancet Oncol. 2018. PMID: 30219628 Clinical Trial.
-
Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial.Lancet Oncol. 2018 May;19(5):603-615. doi: 10.1016/S1470-2045(18)30142-6. Epub 2018 Mar 21. Lancet Oncol. 2018. PMID: 29573941 Clinical Trial.
-
Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
-
Vemurafenib: in unresectable or metastatic melanoma.BioDrugs. 2012 Oct 1;26(5):325-34. doi: 10.2165/11209860-000000000-00000. BioDrugs. 2012. PMID: 22946753 Review.
Cited by
-
Dabrafenib for Treating Unresectable, Advanced or Metastatic BRAF V600 Mutation-Positive Melanoma: An Evidence Review Group Perspective.Pharmacoeconomics. 2015 Sep;33(9):893-904. doi: 10.1007/s40273-015-0276-9. Pharmacoeconomics. 2015. PMID: 25906420 Review.
-
A UK feasibility and validation study of the VE1 monoclonal antibody immunohistochemistry stain for BRAF-V600E mutations in metastatic melanoma.Br J Cancer. 2016 Jul 12;115(2):223-7. doi: 10.1038/bjc.2016.106. Epub 2016 Jun 23. Br J Cancer. 2016. PMID: 27336602 Free PMC article.
-
Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors.J Clin Invest. 2016 May 2;126(5):1834-56. doi: 10.1172/JCI82661. Epub 2016 Apr 4. J Clin Invest. 2016. PMID: 27043285 Free PMC article.
-
The Role of Senescent Cells in Acquired Drug Resistance and Secondary Cancer in BRAFi-Treated Melanoma.Cancers (Basel). 2021 May 7;13(9):2241. doi: 10.3390/cancers13092241. Cancers (Basel). 2021. PMID: 34066966 Free PMC article. Review.
-
Co-clinical assessment identifies patterns of BRAF inhibitor resistance in melanoma.J Clin Invest. 2015 Apr;125(4):1459-70. doi: 10.1172/JCI78954. Epub 2015 Feb 23. J Clin Invest. 2015. PMID: 25705882 Free PMC article.
References
-
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–25. - PubMed
-
- Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–45. - PubMed
-
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

