The roles of αV integrins in lens EMT and posterior capsular opacification
- PMID: 24495224
- PMCID: PMC4000117
- DOI: 10.1111/jcmm.12213
The roles of αV integrins in lens EMT and posterior capsular opacification
Abstract
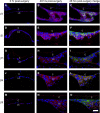
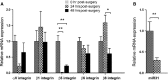
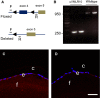
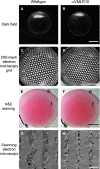
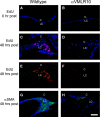
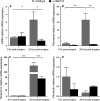
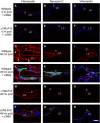
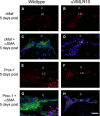
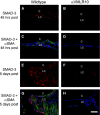
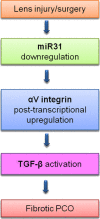
Posterior capsular opacification (PCO) is the major complication arising after cataract treatment. PCO occurs when the lens epithelial cells remaining following surgery (LCs) undergo a wound healing response producing a mixture of α-smooth muscle actin (α-SMA)-expressing myofibroblasts and lens fibre cells, which impair vision. Prior investigations have proposed that integrins play a central role in PCO and we found that, in a mouse fibre cell removal model of cataract surgery, expression of αV integrin and its interacting β-subunits β1, β5, β6, β8 are up-regulated concomitant with α-SMA in LCs following surgery. To test the hypothesis that αV integrins are functionally important in PCO pathogenesis, we created mice lacking the αV integrin subunit in all lens cells. Adult lenses lacking αV integrins are transparent and show no apparent morphological abnormalities when compared with control lenses. However, following surgical fibre cell removal, the LCs in control eyes increased cell proliferation, and up-regulated the expression of α-SMA, β1-integrin, fibronectin, tenascin-C and transforming growth factor beta (TGF-β)-induced protein within 48 hrs, while LCs lacking αV integrins exhibited much less cell proliferation and little to no up-regulation of any of the fibrotic markers tested. This effect appears to result from the known roles of αV integrins in latent TGF-β activation as αV integrin null lenses do not exhibit detectable SMAD-3 phosphorylation after surgery, while this occurs robustly in control lenses, consistent with the known roles for TGF-β in fibrotic PCO. These data suggest that therapeutics antagonizing αV integrin function could be used to prevent fibrotic PCO following cataract surgery.
Keywords: epithelial-to-mesenchymal transition; fibrosis; integrins; lens; posterior capsular opacification; secondary cataract; transforming growth factor beta; wound healing response.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
Fibronectin has multifunctional roles in posterior capsular opacification (PCO).Matrix Biol. 2020 Aug;90:79-108. doi: 10.1016/j.matbio.2020.02.004. Epub 2020 Mar 12. Matrix Biol. 2020. PMID: 32173580 Free PMC article.
-
αVβ8 integrin targeting to prevent posterior capsular opacification.JCI Insight. 2021 Nov 8;6(21):e145715. doi: 10.1172/jci.insight.145715. JCI Insight. 2021. PMID: 34554928 Free PMC article.
-
Histone deacetylase inhibitors trichostatin A and vorinostat inhibit TGFβ2-induced lens epithelial-to-mesenchymal cell transition.Invest Ophthalmol Vis Sci. 2014 Jul 3;55(8):4731-40. doi: 10.1167/iovs.14-14109. Invest Ophthalmol Vis Sci. 2014. PMID: 24994865
-
Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation.Cells Tissues Organs. 2005;179(1-2):43-55. doi: 10.1159/000084508. Cells Tissues Organs. 2005. PMID: 15942192 Review.
-
Roles of TGF β and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity.Int J Mol Sci. 2018 Oct 9;19(10):3093. doi: 10.3390/ijms19103093. Int J Mol Sci. 2018. PMID: 30304871 Free PMC article. Review.
Cited by
-
Aldose reductase inhibition enhances lens regeneration in mice.Chem Biol Interact. 2019 Jul 1;307:58-62. doi: 10.1016/j.cbi.2019.04.021. Epub 2019 Apr 23. Chem Biol Interact. 2019. PMID: 31026421 Free PMC article.
-
Lens Fibrosis: Understanding the Dynamics of Cell Adhesion Signaling in Lens Epithelial-Mesenchymal Transition.Front Cell Dev Biol. 2022 May 17;10:886053. doi: 10.3389/fcell.2022.886053. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35656546 Free PMC article. Review.
-
Development of novel filtering criteria to analyze RNA-sequencing data obtained from the murine ocular lens during embryogenesis.Genom Data. 2014 Dec 1;2:369-374. doi: 10.1016/j.gdata.2014.10.015. Genom Data. 2014. PMID: 25478318 Free PMC article.
-
Biologically Active TNIIIA2 Region in Tenascin-C Molecule: A Major Contributor to Elicit Aggressive Malignant Phenotypes From Tumors/Tumor Stroma.Front Immunol. 2020 Dec 9;11:610096. doi: 10.3389/fimmu.2020.610096. eCollection 2020. Front Immunol. 2020. PMID: 33362799 Free PMC article. Review.
-
Modeling Cataract Surgery in Mice.J Vis Exp. 2023 Dec 1;(202):10.3791/66050. doi: 10.3791/66050. J Vis Exp. 2023. PMID: 38108456 Free PMC article.
References
-
- Asbell PA, Dualan I, Mindel J, et al. Age-related cataract. Lancet. 2005;365:599–609. - PubMed
-
- World Health Organization. Global initiative for the elimination of blindness. WHO/PBL. 2008 ; 97.61. Rev.1.
-
- Steinberg EP, Javitt JC, Sharkey PD, et al. The content and cost of cataract surgery. Arch Ophthalmol. 1993;111:1041–9. - PubMed
-
- Laroche L. actuality in cataract treatment. Rev Prat. 2013;63:43–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

