Thromboxane receptor hyper-responsiveness in hypoxic pulmonary hypertension requires serine 324
- PMID: 24490858
- PMCID: PMC3969080
- DOI: 10.1111/bph.12487
Thromboxane receptor hyper-responsiveness in hypoxic pulmonary hypertension requires serine 324
Abstract
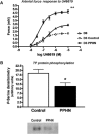
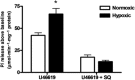
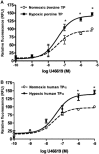
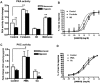
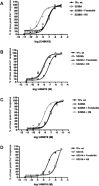
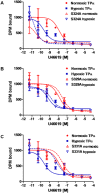
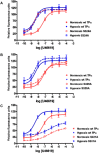
Background and purpose: Dysregulation of the thromboxane A₂ (TP) receptor, resulting in agonist hypersensitivity and hyper-responsiveness, contributes to exaggerated vasoconstriction in the hypoxic pulmonary artery in neonatal persistent pulmonary hypertension. We previously reported that hypoxia inhibits TP receptor phosphorylation, causing desensitization. Hence, we examined the role of PKA-accessible serine residues in determining TP receptor affinity, using site-directed mutational analysis.
Experimental approach: Vasoconstriction to a thromboxane mimetic and phosphorylation of TP receptor serine was examined in pulmonary arteries from neonatal swine with persistent pulmonary hypertension and controls. Effects of hypoxia were determined in porcine and human TP receptors. Human TPα serines at positions 324, 329 and 331 (C-terminal tail) were mutated to alanine and transiently expressed in HEK293T cells. Saturation binding and displacement kinetics of a TP antagonist and agonist were determined in porcine TP, wild-type human TPα and all TP mutants. Agonist-elicited calcium mobilization was determined for each TP mutant, in the presence of a PKA activator or inhibitor, and in hypoxic and normoxic conditions.
Key results: The Ser324A mutant was insensitive to PKA activation and hypoxia, had a high affinity for agonist and increased agonist-induced calcium mobilization. Ser329A was no different from wild-type TP receptors. Ser331A was insensitive to hypoxia and PKA with a decreased agonist-mediated response.
Conclusions and implications: In hypoxic pulmonary hypertension, loss of site-specific phosphorylation of the TP receptor causes agonist hyper-responsiveness. Ser324 is the primary residue phosphorylated by PKA, which regulates TP receptor-agonist interactions. Ser331 mutation confers loss of TP receptor-agonist interaction, regardless of PKA activity.
Keywords: PKA; hypoxia; pulmonary hypertension; thromboxane.
© 2013 The British Pharmacological Society.
Figures
Similar articles
-
Thromboxane hypersensitivity in hypoxic pulmonary artery myocytes: altered TP receptor localization and kinetics.Am J Physiol Lung Cell Mol Physiol. 2007 Mar;292(3):L654-63. doi: 10.1152/ajplung.00229.2006. Epub 2006 Nov 3. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17085527
-
Milrinone attenuates thromboxane receptor-mediated hyperresponsiveness in hypoxic pulmonary arterial myocytes.Br J Pharmacol. 2011 Jul;163(6):1223-36. doi: 10.1111/j.1476-5381.2011.01306.x. Br J Pharmacol. 2011. PMID: 21385177 Free PMC article.
-
Palmitoylation of Gαq determines its association with the thromboxane receptor in hypoxic pulmonary hypertension.Am J Respir Cell Mol Biol. 2014 Jan;50(1):135-43. doi: 10.1165/rcmb.2013-0085OC. Am J Respir Cell Mol Biol. 2014. PMID: 23962128
-
Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology.Pharmacol Ther. 2008 Apr;118(1):18-35. doi: 10.1016/j.pharmthera.2008.01.001. Epub 2008 Jan 26. Pharmacol Ther. 2008. PMID: 18374420 Review.
-
Cell signalling through thromboxane A2 receptors.Cell Signal. 2004 May;16(5):521-33. doi: 10.1016/j.cellsig.2003.10.008. Cell Signal. 2004. PMID: 14751539 Review.
Cited by
-
Enhanced NO-dependent pulmonary vasodilation limits increased vasoconstrictor sensitivity in neonatal chronic hypoxia.Am J Physiol Heart Circ Physiol. 2017 Oct 1;313(4):H828-H838. doi: 10.1152/ajpheart.00123.2017. Epub 2017 Jul 21. Am J Physiol Heart Circ Physiol. 2017. PMID: 28733445 Free PMC article.
-
Nitric oxide augments signaling for contraction in hypoxic pulmonary arterial smooth muscle-Implications for hypoxic pulmonary hypertension.Front Physiol. 2023 Mar 29;14:1144574. doi: 10.3389/fphys.2023.1144574. eCollection 2023. Front Physiol. 2023. PMID: 37064915 Free PMC article.
-
Thromboxane-induced contractile response of mesenteric arterioles is diminished in the older rats and the older hypertensive rats.Front Pharmacol. 2022 Oct 12;13:1019511. doi: 10.3389/fphar.2022.1019511. eCollection 2022. Front Pharmacol. 2022. PMID: 36313372 Free PMC article.
-
Effect of pulsatile stretch on unfolded protein response in a new model of the pulmonary hypertensive vascular wall.Biochem Biophys Rep. 2021 Jul 22;27:101080. doi: 10.1016/j.bbrep.2021.101080. eCollection 2021 Sep. Biochem Biophys Rep. 2021. PMID: 34368469 Free PMC article.
-
Effects of Post-translational Modifications on Membrane Localization and Signaling of Prostanoid GPCR-G Protein Complexes and the Role of Hypoxia.J Membr Biol. 2019 Oct;252(4-5):509-526. doi: 10.1007/s00232-019-00091-4. Epub 2019 Sep 4. J Membr Biol. 2019. PMID: 31485700 Review.
References
-
- Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. - PubMed
-
- Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003;93:656–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

