Investigation of intramolecular dynamics and conformations of α-, β- and γ-synuclein
- PMID: 24489820
- PMCID: PMC3904966
- DOI: 10.1371/journal.pone.0086983
Investigation of intramolecular dynamics and conformations of α-, β- and γ-synuclein
Abstract
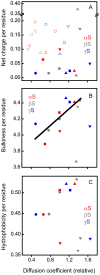
The synucleins are a family of natively unstructured proteins consisting of α-, β-, and γ-synuclein which are primarily expressed in neurons. They have been linked to a wide variety of pathologies, including neurological disorders, such as Parkinson's disease (α-synuclein) and dementia with Lewy bodies (α- and β-synuclein), as well as various types of cancers (γ-synuclein). Self-association is a key pathological feature of many of these disorders, with α-synuclein having the highest propensity to form aggregates, while β-synuclein is the least prone. Here, we used a combination of fluorescence correlation spectroscopy and single molecule Förster resonance energy transfer to compare the intrinsic dynamics of different regions of all three synuclein proteins to investigate any correlation with putative functional or dysfunctional interactions. Despite a relatively high degree of sequence homology, we find that individual regions sample a broad range of diffusion coefficients, differing by almost a factor of four. At low pH, a condition that accelerates aggregation of α-synuclein, on average smaller diffusion coefficients are measured, supporting a hypothesis that slower intrachain dynamics may be correlated with self-association. Moreover, there is a surprising inverse correlation between dynamics and bulkiness of the segments. Aside from this observation, we could not discern any clear relationship between the physico-chemical properties of the constructs and their intrinsic dynamics. This work suggests that while protein dynamics may play a role in modulating self-association or interactions with other binding partners, other factors, particularly the local cellular environment, may be more important.
Conflict of interest statement
Figures

Similar articles
-
Comparative Analysis of the Conformation, Aggregation, Interaction, and Fibril Morphologies of Human α-, β-, and γ-Synuclein Proteins.Biochemistry. 2018 Jul 3;57(26):3830-3848. doi: 10.1021/acs.biochem.8b00343. Epub 2018 Jun 13. Biochemistry. 2018. PMID: 29851342
-
Residual structure, backbone dynamics, and interactions within the synuclein family.J Mol Biol. 2007 Sep 21;372(3):689-707. doi: 10.1016/j.jmb.2007.07.008. Epub 2007 Jul 17. J Mol Biol. 2007. PMID: 17681534 Free PMC article.
-
Excess membrane binding of monomeric alpha-, beta- and gamma-synuclein is invariably associated with inclusion formation and toxicity.Hum Mol Genet. 2021 Nov 16;30(23):2332-2346. doi: 10.1093/hmg/ddab188. Hum Mol Genet. 2021. PMID: 34254125 Free PMC article.
-
Synucleins and their relationship to Parkinson's disease.Cell Tissue Res. 2004 Oct;318(1):163-74. doi: 10.1007/s00441-004-0921-7. Epub 2004 Jul 24. Cell Tissue Res. 2004. PMID: 15503152 Review.
-
The synucleins.Genome Biol. 2002;3(1):REVIEWS3002. doi: 10.1186/gb-2001-3-1-reviews3002. Epub 2001 Dec 20. Genome Biol. 2002. PMID: 11806835 Free PMC article. Review.
Cited by
-
Synucleins: New Data on Misfolding, Aggregation and Role in Diseases.Biomedicines. 2022 Dec 13;10(12):3241. doi: 10.3390/biomedicines10123241. Biomedicines. 2022. PMID: 36551997 Free PMC article. Review.
-
α-Synuclein's Uniquely Long Amphipathic Helix Enhances its Membrane Binding and Remodeling Capacity.J Membr Biol. 2017 Apr;250(2):183-193. doi: 10.1007/s00232-017-9946-1. Epub 2017 Feb 26. J Membr Biol. 2017. PMID: 28239748 Free PMC article.
-
Rethinking gene regulatory networks in light of alternative splicing, intrinsically disordered protein domains, and post-translational modifications.Front Cell Dev Biol. 2015 Feb 26;3:8. doi: 10.3389/fcell.2015.00008. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 25767796 Free PMC article.
-
Alpha and Beta Synucleins: From Pathophysiology to Clinical Application as Biomarkers.Mov Disord. 2022 Apr;37(4):669-683. doi: 10.1002/mds.28941. Epub 2022 Feb 5. Mov Disord. 2022. PMID: 35122299 Free PMC article. Review.
-
Expression of SNCG, MAP2, SDF-1 and CXCR4 in gastric adenocarcinoma and their clinical significance.Int J Clin Exp Pathol. 2014 Sep 15;7(10):6606-15. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25400739 Free PMC article.
References
-
- Lavedan C (1998) The synuclein family. Genome Res 8: 871–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

