The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse
- PMID: 24484975
- PMCID: PMC4084610
- DOI: 10.1016/B978-0-12-420118-7.00002-0
The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse
Abstract
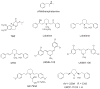
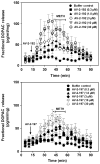
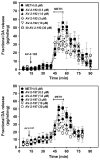
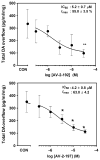
Methamphetamine abuse escalates, but no approved therapeutics are available to treat addicted individuals. Methamphetamine increases extracellular dopamine in reward-relevant pathways by interacting at vesicular monoamine transporter-2 (VMAT2) to inhibit dopamine uptake and promote dopamine release from synaptic vesicles, increasing cytosolic dopamine available for reverse transport by the dopamine transporter (DAT). VMAT2 is the target of our iterative drug discovery efforts to identify pharmacotherapeutics for methamphetamine addiction. Lobeline, the major alkaloid in Lobelia inflata, potently inhibited VMAT2, methamphetamine-evoked striatal dopamine release, and methamphetamine self-administration in rats but exhibited high affinity for nicotinic acetylcholine receptors (nAChRs). Defunctionalized, unsaturated lobeline analog, meso-transdiene (MTD), exhibited lobeline-like in vitro pharmacology, lacked nAChR affinity, but exhibited high affinity for DAT, suggesting potential abuse liability. The 2,4-dicholorophenyl MTD analog, UKMH-106, exhibited selectivity for VMAT2 over DAT, inhibited methamphetamine-evoked dopamine release, but required a difficult synthetic approach. Lobelane, a saturated, defunctionalized lobeline analog, inhibited the neurochemical and behavioral effects of methamphetamine; tolerance developed to the lobelane-induced decrease in methamphetamine self-administration. Improved drug-likeness was afforded by the incorporation of a chiral N-1,2-dihydroxypropyl moiety into lobelane to afford GZ-793A, which inhibited the neurochemical and behavioral effects of methamphetamine, without tolerance. From a series of 2,5-disubstituted pyrrolidine analogs, AV-2-192 emerged as a lead, exhibiting high affinity for VMAT2 and inhibiting methamphetamine-evoked dopamine release. Current results support the hypothesis that potent, selective VMAT2 inhibitors provide the requisite preclinical behavioral profile for evaluation as pharmacotherapeutics for methamphetamine abuse and emphasize selectivity for VMAT2 relative to DAT as a criterion for reducing abuse liability of the therapeutic.
Keywords: AV-2-192; GZ-793A; Lobelane; Lobeline; Methamphetamine; VMAT2.
© 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The University of Kentucky holds patents on the compounds described in the current work, some of which have been licensed by Yaupon Therapeutics/Ceptaris Inc. A potential royalty stream to LPD and PAC may occur consistent with the University of Kentucky policy. Both LPD and PAC are founders of, and have financial interest in, Yaupon Therapeutics/Ceptaris Inc.
Figures

Similar articles
-
New Scaffold for Lead Compounds to Treat Methamphetamine Use Disorders.AAPS J. 2018 Feb 9;20(2):29. doi: 10.1208/s12248-018-0192-y. AAPS J. 2018. PMID: 29427069 Free PMC article.
-
Novel N-1,2-dihydroxypropyl analogs of lobelane inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release.J Pharmacol Exp Ther. 2011 Oct;339(1):286-97. doi: 10.1124/jpet.111.184770. Epub 2011 Jul 21. J Pharmacol Exp Ther. 2011. PMID: 21778282 Free PMC article.
-
Lobelane inhibits methamphetamine-evoked dopamine release via inhibition of the vesicular monoamine transporter-2.J Pharmacol Exp Ther. 2010 Feb;332(2):612-21. doi: 10.1124/jpet.109.160275. Epub 2009 Oct 23. J Pharmacol Exp Ther. 2010. PMID: 19855096 Free PMC article.
-
Design, synthesis and interaction at the vesicular monoamine transporter-2 of lobeline analogs: potential pharmacotherapies for the treatment of psychostimulant abuse.Curr Top Med Chem. 2011;11(9):1103-27. doi: 10.2174/156802611795371332. Curr Top Med Chem. 2011. PMID: 21050177 Free PMC article. Review.
-
A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse.Biochem Pharmacol. 2002 Jan 15;63(2):89-98. doi: 10.1016/s0006-2952(01)00899-1. Biochem Pharmacol. 2002. PMID: 11841781 Review.
Cited by
-
Current status and future directions for a neurotoxicity hazard assessment framework that integrates in silico approaches.Comput Toxicol. 2022 May;22:100223. doi: 10.1016/j.comtox.2022.100223. Epub 2022 Mar 17. Comput Toxicol. 2022. PMID: 35844258 Free PMC article.
-
Nucleus accumbens hyperpolarization-activated cyclic nucleotide-gated channels modulate methamphetamine self-administration in rats.Psychopharmacology (Berl). 2016 Aug;233(15-16):3017-29. doi: 10.1007/s00213-016-4349-z. Epub 2016 Jun 22. Psychopharmacology (Berl). 2016. PMID: 27329413
-
Argon blocks the expression of locomotor sensitization to amphetamine through antagonism at the vesicular monoamine transporter-2 and mu-opioid receptor in the nucleus accumbens.Transl Psychiatry. 2015 Jul 7;5(7):e594. doi: 10.1038/tp.2015.27. Transl Psychiatry. 2015. PMID: 26151922 Free PMC article.
-
SLC transporters as therapeutic targets: emerging opportunities.Nat Rev Drug Discov. 2015 Aug;14(8):543-60. doi: 10.1038/nrd4626. Epub 2015 Jun 26. Nat Rev Drug Discov. 2015. PMID: 26111766 Free PMC article. Review.
-
GZ-793A inhibits the neurochemical effects of methamphetamine via a selective interaction with the vesicular monoamine transporter-2.Eur J Pharmacol. 2017 Jan 15;795:143-149. doi: 10.1016/j.ejphar.2016.12.016. Epub 2016 Dec 13. Eur J Pharmacol. 2017. PMID: 27986625 Free PMC article.
References
-
- Andersen PH. Biochemical and pharmacological characterization of [3H]GBR 12935 binding in vitro to rat striatal membranes: Labeling of the dopamine uptake complex. Journal of Neurochemistry. 1987;48:1887–1896. - PubMed
-
- Ary TE, Komiskey HL. Phencyclidine: Effect on the accumulation of 3H-dopamine in synaptic vesicles. Life Sciences. 1980;26:575–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

