Activation of duck RIG-I by TRIM25 is independent of anchored ubiquitin
- PMID: 24466302
- PMCID: PMC3900705
- DOI: 10.1371/journal.pone.0086968
Activation of duck RIG-I by TRIM25 is independent of anchored ubiquitin
Abstract
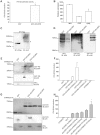
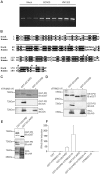
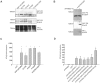
Retinoic acid inducible gene I (RIG-I) is a viral RNA sensor crucial in defense against several viruses including measles, influenza A and hepatitis C. RIG-I activates type-I interferon signalling through the adaptor for mitochondrial antiviral signaling (MAVS). The E3 ubiquitin ligase, tripartite motif containing protein 25 (TRIM25), activates human RIG-I through generation of anchored K63-linked polyubiquitin chains attached to lysine 172, or alternatively, through the generation of unanchored K63-linked polyubiquitin chains that interact non-covalently with RIG-I CARD domains. Previously, we identified RIG-I of ducks, of interest because ducks are the host and natural reservoir of influenza viruses, and showed it initiates innate immune signaling leading to production of interferon-beta (IFN-β). We noted that K172 is not conserved in RIG-I of ducks and other avian species, or mouse. Because K172 is important for both mechanisms of activation of human RIG-I, we investigated whether duck RIG-I was activated by TRIM25, and if other residues were the sites for attachment of ubiquitin. Here we show duck RIG-I CARD domains are ubiquitinated for activation, and ubiquitination depends on interaction with TRIM25, as a splice variant that cannot interact with TRIM25 is not ubiquitinated, and cannot be activated. We expressed GST-fusion proteins of duck CARD domains and characterized TRIM25 modifications of CARD domains by mass spectrometry. We identified two sites that are ubiquitinated in duck CARD domains, K167 and K193, and detected K63 linked polyubiquitin chains. Site directed mutagenesis of each site alone, does not alter the ubiquitination profile of the duck CARD domains. However, mutation of both sites resulted in loss of all attached ubiquitin and polyubiquitin chains. Remarkably, the double mutant duck RIG-I CARD still interacts with TRIM25, and can still be activated. Our results demonstrate that anchored ubiquitin chains are not necessary for TRIM25 activation of duck RIG-I.
Conflict of interest statement
Figures

Similar articles
-
Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction.Proc Natl Acad Sci U S A. 2008 Oct 28;105(43):16743-8. doi: 10.1073/pnas.0804947105. Epub 2008 Oct 23. Proc Natl Acad Sci U S A. 2008. PMID: 18948594 Free PMC article.
-
The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25.Sci Signal. 2014 Jan 7;7(307):ra3. doi: 10.1126/scisignal.2004577. Sci Signal. 2014. PMID: 24399297 Free PMC article.
-
Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein.PLoS Pathog. 2012;8(11):e1003059. doi: 10.1371/journal.ppat.1003059. Epub 2012 Nov 29. PLoS Pathog. 2012. PMID: 23209422 Free PMC article.
-
Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review.
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
Cited by
-
Avian Pattern Recognition Receptor Sensing and Signaling.Vet Sci. 2020 Jan 27;7(1):14. doi: 10.3390/vetsci7010014. Vet Sci. 2020. PMID: 32012730 Free PMC article. Review.
-
Differential proteome response to H5N1 highly pathogenic avian influenza (HPAI) viruses infection in duck.Front Immunol. 2022 Aug 19;13:965454. doi: 10.3389/fimmu.2022.965454. eCollection 2022. Front Immunol. 2022. PMID: 36059479 Free PMC article.
-
Avian Influenza NS1 Proteins Inhibit Human, but Not Duck, RIG-I Ubiquitination and Interferon Signaling.J Virol. 2022 Sep 28;96(18):e0077622. doi: 10.1128/jvi.00776-22. Epub 2022 Sep 7. J Virol. 2022. PMID: 36069546 Free PMC article.
-
Duck RIG-I CARD Domain Induces the Chicken IFN-β by Activating NF-κB.Biomed Res Int. 2015;2015:348792. doi: 10.1155/2015/348792. Epub 2015 Mar 30. Biomed Res Int. 2015. PMID: 25918711 Free PMC article.
-
Molecular Evolution of the Influenza A Virus Non-structural Protein 1 in Interspecies Transmission and Adaptation.Front Microbiol. 2021 Oct 4;12:693204. doi: 10.3389/fmicb.2021.693204. eCollection 2021. Front Microbiol. 2021. PMID: 34671321 Free PMC article. Review.
References
-
- Schlee M, Hartmann E, Coch C, Wimmenauer V, Janke M, et al. (2009) Approaching the RNA ligand for RIG-I? Immunol Rev 227: 66–74. - PubMed
-
- Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, et al. (2011) Structural Basis for the Activation of Innate Immune Pattern-Recognition Receptor RIG-I by Viral RNA. Cell 147: 423–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

