Interplay between phosphoinositide lipids and calcium signals at the leading edge of chemotaxing ameboid cells
- PMID: 24451847
- PMCID: PMC4104151
- DOI: 10.1016/j.chemphyslip.2014.01.002
Interplay between phosphoinositide lipids and calcium signals at the leading edge of chemotaxing ameboid cells
Abstract
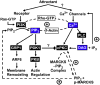
The chemotactic migration of eukaryotic ameboid cells up concentration gradients is among the most advanced forms of cellular behavior. Chemotaxis is controlled by a complex network of signaling proteins bound to specific lipids on the cytoplasmic surface of the plasma membrane at the front of the cell, or the leading edge. The central lipid players in this leading edge signaling pathway include the phosphoinositides PI(4,5)P2 (PIP2) and PI(3,4,5)P3 (PIP3), both of which play multiple roles. The products of PI(4,5)P2 hydrolysis, diacylglycerol (DAG) and Ins(1,4,5)P3 (IP3), are also implicated as important players. Together, these leading edge phosphoinositides and their degradation products, in concert with a local Ca(2+) signal, control the recruitment and activities of many peripheral membrane proteins that are crucial to the leading edge signaling network. The present critical review summarizes the current molecular understanding of chemotactic signaling at the leading edge, including newly discovered roles of phosphoinositide lipids and Ca(2+), while highlighting key questions for future research.
Keywords: C2 domain; PDK1; PH domain; PI3K; PKC; Phosphatidylinositol lipid.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Figures


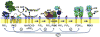
Similar articles
-
A PKC-MARCKS-PI3K regulatory module links Ca2+ and PIP3 signals at the leading edge of polarized macrophages.PLoS One. 2018 May 1;13(5):e0196678. doi: 10.1371/journal.pone.0196678. eCollection 2018. PLoS One. 2018. PMID: 29715315 Free PMC article.
-
PI 3-kinases and PTEN: how opposites chemoattract.Cell. 2002 May 31;109(5):541-4. doi: 10.1016/s0092-8674(02)00765-1. Cell. 2002. PMID: 12062096 Review.
-
The role of phosphoinositide-regulated actin reorganization in chemotaxis and cell migration.Br J Pharmacol. 2014 Dec;171(24):5541-54. doi: 10.1111/bph.12777. Epub 2014 Nov 24. Br J Pharmacol. 2014. PMID: 25420930 Free PMC article. Review.
-
Cholesterol stabilizes fluid phosphoinositide domains.Chem Phys Lipids. 2014 Sep;182:52-61. doi: 10.1016/j.chemphyslip.2014.02.003. Epub 2014 Feb 17. Chem Phys Lipids. 2014. PMID: 24556334 Free PMC article.
-
Phosphatidylinositol (3,4) bisphosphate-specific phosphatases and effector proteins: A distinct branch of PI3K signaling.Cell Signal. 2015 Sep;27(9):1789-98. doi: 10.1016/j.cellsig.2015.05.013. Epub 2015 May 27. Cell Signal. 2015. PMID: 26022180 Review.
Cited by
-
Phospholipase C and D regulation of Src, calcium release and membrane fusion during Xenopus laevis development.Dev Biol. 2015 May 15;401(2):188-205. doi: 10.1016/j.ydbio.2015.02.020. Epub 2015 Mar 5. Dev Biol. 2015. PMID: 25748412 Free PMC article. Review.
-
TRPM2 ion channels steer neutrophils towards a source of hydrogen peroxide.Sci Rep. 2021 Apr 29;11(1):9339. doi: 10.1038/s41598-021-88224-5. Sci Rep. 2021. PMID: 33927223 Free PMC article.
-
Single-molecule studies reveal regulatory interactions between master kinases PDK1, AKT1, and PKC.Biophys J. 2021 Dec 21;120(24):5657-5673. doi: 10.1016/j.bpj.2021.10.015. Epub 2021 Oct 19. Biophys J. 2021. PMID: 34673053 Free PMC article.
-
A PKC-MARCKS-PI3K regulatory module links Ca2+ and PIP3 signals at the leading edge of polarized macrophages.PLoS One. 2018 May 1;13(5):e0196678. doi: 10.1371/journal.pone.0196678. eCollection 2018. PLoS One. 2018. PMID: 29715315 Free PMC article.
-
Kinetic Changes of Ptdins (3,4,5) P3 within Fast and Slow Turnover Rates of Focal Adhesion.Rep Biochem Mol Biol. 2022 Jul;11(2):262-269. doi: 10.52547/rbmb.11.2.262. Rep Biochem Mol Biol. 2022. PMID: 36164635 Free PMC article.
References
-
- Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. - PubMed
-
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. - PubMed
-
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

