The Novel Secreted Adipokine WNT1-inducible Signaling Pathway Protein 2 (WISP2) Is a Mesenchymal Cell Activator of Canonical WNT
- PMID: 24451367
- PMCID: PMC3945351
- DOI: 10.1074/jbc.M113.511964
The Novel Secreted Adipokine WNT1-inducible Signaling Pathway Protein 2 (WISP2) Is a Mesenchymal Cell Activator of Canonical WNT
Abstract
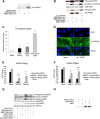
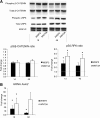
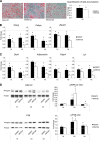
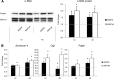
WNT1-inducible-signaling pathway protein 2 (WISP2) is primarily expressed in mesenchymal stem cells, fibroblasts, and adipogenic precursor cells. It is both a secreted and cytosolic protein, the latter regulating precursor cell adipogenic commitment and PPARγ induction by BMP4. To examine the effect of the secreted protein, we expressed a full-length and a truncated, non-secreted WISP2 in NIH3T3 fibroblasts. Secreted, but not truncated WISP2 activated the canonical WNT pathway with increased β-catenin levels, its nuclear targeting phosphorylation, and LRP5/6 phosphorylation. It also inhibited Pparg activation and the effect of secreted WISP2 was reversed by the WNT antagonist DICKKOPF-1. Differentiated 3T3-L1 adipose cells were also target cells where extracellular WISP2 activated the canonical WNT pathway, inhibited Pparg and associated adipose genes and, similar to WNT3a, promoted partial dedifferentiation of the cells and the induction of a myofibroblast phenotype with activation of markers of fibrosis. Thus, WISP2 exerts dual actions in mesenchymal precursor cells; secreted WISP2 activates canonical WNT and maintains the cells in an undifferentiated state, whereas cytosolic WISP2 regulates adipogenic commitment.
Keywords: Adipose Tissue; Beta-catenin; Bone Morphogenetic Protein (BMP); Cell Biology; Obesity; Peroxisome Proliferator-activated receptor (PPAR); Wnt Pathway.
Figures
Similar articles
-
WISP2 regulates preadipocyte commitment and PPARγ activation by BMP4.Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2563-8. doi: 10.1073/pnas.1211255110. Epub 2013 Jan 28. Proc Natl Acad Sci U S A. 2013. PMID: 23359679 Free PMC article.
-
Dickkopf (Dkk)-2 is a beige fat-enriched adipokine to regulate adipogenesis.Biochem Biophys Res Commun. 2021 Apr 9;548:211-216. doi: 10.1016/j.bbrc.2021.02.068. Epub 2021 Feb 26. Biochem Biophys Res Commun. 2021. PMID: 33647798
-
Dickkopf-1 promotes the differentiation and adipocytokines secretion via canonical Wnt signaling pathway in primary cultured human preadipocytes.Obes Res Clin Pract. 2016 Jul-Aug;10(4):454-64. doi: 10.1016/j.orcp.2015.08.016. Epub 2015 Sep 14. Obes Res Clin Pract. 2016. PMID: 26383960
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
Role of CCN5 (WNT1 inducible signaling pathway protein 2) in pancreatic islets.J Diabetes. 2017 May;9(5):462-474. doi: 10.1111/1753-0407.12507. Epub 2016 Dec 26. J Diabetes. 2017. PMID: 27863006 Review.
Cited by
-
Accumulation of 4-Hydroxynonenal Characterizes Diabetic Fat and Modulates Adipogenic Differentiation of Adipose Precursor Cells.Int J Mol Sci. 2023 Nov 23;24(23):16645. doi: 10.3390/ijms242316645. Int J Mol Sci. 2023. PMID: 38068967 Free PMC article.
-
Mandibular undifferentiated pleomorphic sarcoma: Molecular analysis of a primary cell population.Clin Exp Dent Res. 2020 Oct;6(5):495-505. doi: 10.1002/cre2.301. Epub 2020 Jul 11. Clin Exp Dent Res. 2020. PMID: 32652895 Free PMC article.
-
Adipose Morphology: a Critical Factor in Regulation of Human Metabolic Diseases and Adipose Tissue Dysfunction.Obes Surg. 2020 Dec;30(12):5086-5100. doi: 10.1007/s11695-020-04983-6. Epub 2020 Oct 6. Obes Surg. 2020. PMID: 33021706 Free PMC article. Review.
-
Single-cell RNA-seq reveals intratumoral heterogeneity in osteosarcoma patients: A review.J Bone Oncol. 2023 Mar 20;39:100475. doi: 10.1016/j.jbo.2023.100475. eCollection 2023 Apr. J Bone Oncol. 2023. PMID: 37034356 Free PMC article. Review.
-
The fat cell epigenetic signature in post-obese women is characterized by global hypomethylation and differential DNA methylation of adipogenesis genes.Int J Obes (Lond). 2015 Jun;39(6):910-9. doi: 10.1038/ijo.2015.31. Epub 2015 Mar 18. Int J Obes (Lond). 2015. PMID: 25783037
References
-
- Després J. P., Lemieux I., Bergeron J., Pibarot P., Mathieu P., Larose E., Rodés-Cabau J., Bertrand O. F., Poirier P. (2008) Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28, 1039–1049 - PubMed
-
- Virtue S., Vidal-Puig A. (2010) Adipose tissue expandability, lipotoxicity and the metabolic syndrome–an allostatic perspective. Biochim. Biophys. Acta 1801, 338–349 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

