PKA phosphorylation reshapes the pharmacological kinetics of BmK AS, a unique site-4 sodium channel-specific modulator
- PMID: 24430351
- PMCID: PMC5379197
- DOI: 10.1038/srep03721
PKA phosphorylation reshapes the pharmacological kinetics of BmK AS, a unique site-4 sodium channel-specific modulator
Abstract
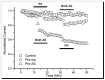
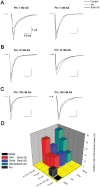
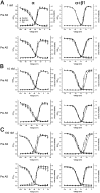
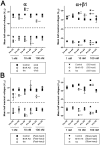
Although modulation of the activity of voltage-gated sodium channels (VGSCs) by protein kinase A (PKA) phosphorylation has been investigated in multiple preparations, the pharmacological sensitivity of VGSCs to scorpion toxins after PKA phosphorylation has rarely been approached. In this study, the effects of BmK AS, a sodium channel-specific modulator from Chinese scorpion Buthus martensi Karsch, on the voltage-dependent activation and inactivation of Nav1.2 were examined before and after PKA activation. After PKA phosphorylation, the pattern of dose-dependent modulation of BmK AS, on both Nav1.2α and Nav1.2 (α + β1) was reshaped. Meanwhile, the shifts in voltage-dependency of activation and inactivation induced by BmK AS were attenuated. The results suggested that PKA might play a role in different patterns how β-like toxins such as BmK AS modulate gating properties and peak currents of VGSCs.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Chinese-scorpion (Buthus martensi Karsch) toxin BmK alphaIV, a novel modulator of sodium channels: from genomic organization to functional analysis.Biochem J. 2006 Nov 1;399(3):445-53. doi: 10.1042/BJ20060035. Biochem J. 2006. PMID: 16800812 Free PMC article.
-
U-shaped dose-dependent effects of BmK AS, a unique scorpion polypeptide toxin, on voltage-gated sodium channels.Br J Pharmacol. 2009 Dec;158(8):1895-903. doi: 10.1111/j.1476-5381.2009.00471.x. Br J Pharmacol. 2009. PMID: 19912232 Free PMC article.
-
Scorpion toxin BmK I directly activates Nav1.8 in primary sensory neurons to induce neuronal hyperexcitability in rats.Protein Cell. 2015 Jun;6(6):443-52. doi: 10.1007/s13238-015-0154-4. Epub 2015 Apr 24. Protein Cell. 2015. PMID: 25903152 Free PMC article.
-
Exploring the obscure profiles of pharmacological binding sites on voltage-gated sodium channels by BmK neurotoxins.Protein Cell. 2011 Jun;2(6):437-44. doi: 10.1007/s13238-011-1064-8. Epub 2011 Jul 12. Protein Cell. 2011. PMID: 21748593 Free PMC article. Review.
-
Anti-epileptic/pro-epileptic effects of sodium channel modulators from Buthus martensii Karsch.Sheng Li Xue Bao. 2022 Aug 25;74(4):621-632. Sheng Li Xue Bao. 2022. PMID: 35993213 Review.
Cited by
-
Activation of protein kinase A in the amygdala modulates anxiety-like behaviors in social defeat exposed mice.Mol Brain. 2016 Jan 8;9:3. doi: 10.1186/s13041-015-0181-3. Mol Brain. 2016. PMID: 26747511 Free PMC article.
References
-
- Mao J., Mayer D. J., Hayes R. L. & Price D. D. Spatial patterns of increased spinal cord membrane-bound protein kinase C and their relation to increases in 14C-2-deoxyglucose metabolic activity in rats with painful peripheral mononeuropathy. J Neurophysiol 70, 470–481 (1993). - PubMed
-
- Vijayaragavan K., Boutjdir M. & Chahine M. Modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by protein kinase A and protein kinase C. J Neurophysiol 91, 1556–1569 (2004). - PubMed
-
- Murphy B. J., Rogers J., Perdichizzi A. P., Colvin A. A. & Catterall W. A. cAMP-dependent phosphorylation of two sites in the alpha subunit of the cardiac sodium channel. J Biol Chem 271, 28837–28843 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

