In vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer models
- PMID: 24430235
- PMCID: PMC3949200
- DOI: 10.1038/gt.2013.84
In vivo safety, biodistribution and antitumor effects of uPAR retargeted oncolytic measles virus in syngeneic cancer models
Abstract
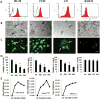
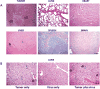
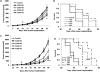
The urokinase receptor (uPAR) is a clinically relevant target for novel biological therapies. We have previously rescued oncolytic measles viruses fully retargeted against human (MV-h-uPA) or murine (MV-m-uPA) uPAR. Here, we investigated the in vivo effects of systemic administration of MV-m-uPA in immunocompetent cancer models. MV-m-uPA induced in vitro cytotoxicity and replicated in a receptor-dependent manner in murine mammary (4T1) and colon (MC-38 and CT-26) cancer cells. Intravenous administration of MV-m-uPA to 4T1 tumor-bearing mice was not associated with significant clinical or laboratory toxicity. Higher MV-N RNA copy numbers were detected in primary tumors, and viable viral particles were recovered from tumor-bearing tissues only. Non-tumor-bearing organs did not show histological signs of viral-induced toxicity. Serum anti-MV antibodies were detected at day 14 of treatment. Immunohistochemistry and immunofluorescence studies confirmed successful tumor targeting and demonstrated enhanced MV-m-uPA-induced tumor cell apoptosis in treated compared with control mice. Significant antitumor effects and prolonged survival were observed after systemic administration of MV-m-uPA in colon (CT-26) and mammary (4T1) cancer models. The above results show safety and feasibility of uPAR targeting by an oncolytic virus, and confirm significant antitumor effects in highly aggressive syngeneic immunocompetent cancer models.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures


Similar articles
-
In vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancer.Breast Cancer Res Treat. 2015 Jan;149(1):99-108. doi: 10.1007/s10549-014-3236-8. Epub 2014 Dec 18. Breast Cancer Res Treat. 2015. PMID: 25519042 Free PMC article.
-
Tumor and vascular targeting of a novel oncolytic measles virus retargeted against the urokinase receptor.Cancer Res. 2009 Feb 15;69(4):1459-68. doi: 10.1158/0008-5472.CAN-08-2628. Epub 2009 Feb 10. Cancer Res. 2009. PMID: 19208845 Free PMC article.
-
Molecular Effects of Stromal-Selective Targeting by uPAR-Retargeted Oncolytic Virus in Breast Cancer.Mol Cancer Res. 2017 Oct;15(10):1410-1420. doi: 10.1158/1541-7786.MCR-17-0016. Epub 2017 Jul 5. Mol Cancer Res. 2017. PMID: 28679779 Free PMC article.
-
Therapeutic potential of oncolytic measles virus: promises and challenges.Clin Pharmacol Ther. 2010 Nov;88(5):620-5. doi: 10.1038/clpt.2010.211. Epub 2010 Sep 29. Clin Pharmacol Ther. 2010. PMID: 20881957 Review.
-
Measles virus for cancer therapy.Curr Top Microbiol Immunol. 2009;330:213-41. doi: 10.1007/978-3-540-70617-5_11. Curr Top Microbiol Immunol. 2009. PMID: 19203112 Free PMC article. Review.
Cited by
-
Oncolytic Virotherapy: From Bench to Bedside.Front Cell Dev Biol. 2021 Nov 26;9:790150. doi: 10.3389/fcell.2021.790150. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901031 Free PMC article. Review.
-
Investigating the potential of oncolytic viruses for cancer treatment via MSC delivery.Cell Commun Signal. 2023 Sep 4;21(1):228. doi: 10.1186/s12964-023-01232-y. Cell Commun Signal. 2023. PMID: 37667271 Free PMC article. Review.
-
Urokinase-Type Plasminogen Activator Receptor (uPAR) in Inflammation and Disease: A Unique Inflammatory Pathway Activator.Biomedicines. 2024 May 24;12(6):1167. doi: 10.3390/biomedicines12061167. Biomedicines. 2024. PMID: 38927374 Free PMC article. Review.
-
Immunostimulatory bacterial antigen-armed oncolytic measles virotherapy significantly increases the potency of anti-PD1 checkpoint therapy.J Clin Invest. 2021 Jul 1;131(13):e141614. doi: 10.1172/JCI141614. J Clin Invest. 2021. PMID: 34196308 Free PMC article.
-
Preparation, Pharmacokinetics, and Antitumor Potential of Miltefosine-Loaded Nanostructured Lipid Carriers.Int J Nanomedicine. 2021 May 11;16:3255-3273. doi: 10.2147/IJN.S299443. eCollection 2021. Int J Nanomedicine. 2021. Retraction in: Int J Nanomedicine. 2023 Mar 01;18:1107-1108. doi: 10.2147/IJN.S409956 PMID: 34012260 Free PMC article. Retracted.
References
-
- Patel MR, Kratzke RA. Oncolytic virus therapy for cancer: the first wave of translational clinical trials. Translational research : the journal of laboratory and clinical medicine. 2013;161:355–64. - PubMed
-
- Nakamura T, Russell SJ. Oncolytic measles viruses for cancer therapy. Expert Opin Biol Ther. 2004;4:1685–92. - PubMed
-
- Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002;9:961–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

