Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme
- PMID: 24423086
- PMCID: PMC4029467
- DOI: 10.1186/1756-9966-32-82
Clinical experience with ipilimumab 10 mg/kg in patients with melanoma treated at Italian centres as part of a European expanded access programme
Abstract
Background: Patients with advanced melanoma are faced with a poor prognosis and, until recently, limited treatment options. Ipilimumab, a novel immunotherapy that blocks cytotoxic T-lymphocyte-associated antigen-4, was the first agent to improve survival of patients with advanced melanoma in a randomised, controlled phase 3 trial. We used data from an expanded access programme (EAP) at Italian centres to evaluate the clinical activity and safety profile of ipilimumab 10 mg/kg in patients with advanced melanoma in a setting more similar to that of daily practice.
Methods: Data were collected from patients enrolled in an ipilimumab EAP across eight participating Italian centres. As per the EAP protocol, patients had life-threatening, unresectable stage III/IV melanoma, had failed or did not tolerate previous treatments and had no other therapeutic option available. Treatment comprised ipilimumab 10 mg/kg every 3 weeks for a total of four doses. If physicians believed patients would continue to derive benefit from ipilimumab treatment, maintenance therapy with ipilimumab 10 mg/kg was provided every 12 weeks. Tumour responses were assessed every 12 weeks using modified World Health Organization criteria and safety continuously monitored.
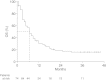
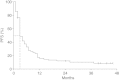
Results: Seventy-four pretreated patients with advanced melanoma were treated with ipilimumab 10 mg/kg. Of these, 9 (13.0%) had an objective response, comprising 3 patients with a complete response and 6 with a partial response. Median overall survival was 7.0 months (95% confidence interval, 5.3-8.7) and 16.6% of patients were alive after 3 years. Forty-five patients (60.8%) reported treatment-related adverse events of any grade, which were most commonly low-grade pruritus, pain, fever and diarrhoea. Grade 3 or 4 treatment-related AEs were reported in 8 patients (10.8%).
Conclusions: The clinical activity and safety profile of ipilimumab 10 mg/kg in the EAP was similar to that seen in previous clinical trials of ipilimumab in pretreated patient populations.
Figures
Similar articles
-
Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme.J Exp Clin Cancer Res. 2014 Apr 4;33(1):30. doi: 10.1186/1756-9966-33-30. J Exp Clin Cancer Res. 2014. PMID: 24708900 Free PMC article. Clinical Trial.
-
Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma.Eur J Cancer. 2014 Jan;50(1):121-7. doi: 10.1016/j.ejca.2013.09.007. Epub 2013 Oct 4. Eur J Cancer. 2014. PMID: 24100024
-
Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy).Cancer Immunol Immunother. 2011 Apr;60(4):467-77. doi: 10.1007/s00262-010-0958-2. Epub 2010 Dec 18. Cancer Immunol Immunother. 2011. PMID: 21170646 Free PMC article. Clinical Trial.
-
Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer.Pharmacol Res. 2012 Jan;65(1):9-22. doi: 10.1016/j.phrs.2011.09.002. Epub 2011 Sep 10. Pharmacol Res. 2012. PMID: 21930211 Review.
-
Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma.Ann N Y Acad Sci. 2013 Jul;1291(1):1-13. doi: 10.1111/nyas.12180. Epub 2013 Jun 17. Ann N Y Acad Sci. 2013. PMID: 23772560 Free PMC article. Review.
Cited by
-
BAP31, a promising target for the immunotherapy of malignant melanomas.J Exp Clin Cancer Res. 2015 Apr 18;34(1):36. doi: 10.1186/s13046-015-0153-6. J Exp Clin Cancer Res. 2015. PMID: 25903101 Free PMC article.
-
The Current Status of Immune Checkpoint Inhibitors in Neuro-Oncology: A Systematic Review.Cancers (Basel). 2020 Mar 4;12(3):586. doi: 10.3390/cancers12030586. Cancers (Basel). 2020. PMID: 32143288 Free PMC article. Review.
-
Ipilimumab in Pretreated Patients With Advanced Malignant Melanoma: Results of the South African Expanded-Access Program.J Glob Oncol. 2016 Nov 2;3(5):515-523. doi: 10.1200/JGO.2016.006544. eCollection 2017 Oct. J Glob Oncol. 2016. PMID: 29094091 Free PMC article.
-
Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications.Oncoimmunology. 2015 Mar 2;4(4):e1008814. doi: 10.1080/2162402X.2015.1008814. eCollection 2015 Apr. Oncoimmunology. 2015. PMID: 26137403 Free PMC article. Review.
-
Effects of BRAF mutations and BRAF inhibition on immune responses to melanoma.Mol Cancer Ther. 2014 Dec;13(12):2769-83. doi: 10.1158/1535-7163.MCT-14-0290. Epub 2014 Nov 10. Mol Cancer Ther. 2014. PMID: 25385327 Free PMC article. Review.
References
-
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;32:6199–6206. doi: 10.1200/JCO.2009.23.4799. - DOI - PMC - PubMed
-
- Maio M, Ascierto P, Testori A, Ridolfi R, Bajetta E, Queirolo P, Guida M, Romanini A, Chiarion-Sileni V, Pigozzo J, Di Giacomo AM, Calandriello M, Didoni G, van Baardewijk M, Konto C, Lucioni C. The cost of unresectable stage III or stage IV melanoma in Italy. J Exp Clin Cancer Res. 2012;32:91–103. doi: 10.1186/1756-9966-31-91. - DOI - PMC - PubMed
-
- Dummer R, Guggenheim M, Arnold AW, Braun R, von Moos R. Project Group Melanoma of the Swiss Group for Clinical Cancer Research. Updated swiss guidelines for the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly. 2011;32:w13320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

