Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases
- PMID: 24421755
- PMCID: PMC3872787
- DOI: 10.3389/fncel.2013.00275
Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases
Abstract
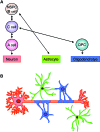
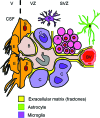
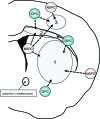
White matter dysfunction is an important part of many CNS disorders including multiple sclerosis (MS) and vascular dementia. Within injured areas, myelin loss and oligodendrocyte death may trigger endogenous attempts at regeneration. However, during disease progression, remyelination failure may eventually occur due to impaired survival/proliferation, migration/recruitment, and differentiation of oligodendrocyte precursor cells (OPCs). The ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ) are the main sources of neural stem/progenitor cells (NSPCs), which can give rise to neurons as well as OPCs. Under normal conditions in the adult brain, the V-SVZ progenitors generate a large number of neurons with a small number of oligodendrocyte lineage cells. However, after demyelination, the fate of V-SVZ-derived progenitor cells shifts from neurons to OPCs, and these newly generated OPCs migrate to the demyelinating lesions to ease white matter damage. In this mini-review, we will summarize the recent studies on extrinsic (e.g., vasculature, extracellular matrix (ECM), cerebrospinal fluid (CSF)) and intrinsic (e.g., transcription factors, epigenetic modifiers) factors, which mediate oligodendrocyte generation from the V-SVZ progenitor cells. A deeper understanding of the mechanisms that regulate the fate of V-SVZ progenitor cells may lead to new therapeutic approaches for ameliorating white matter dysfunction and damage in CNS disorders.
Keywords: demyelination; multiple sclerosis; neural stem/progenitor cells; oligodendrocyte precursor cells; oligodendrogenesis; subventricular zone; vascular dementia.
Figures
Similar articles
-
Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination.J Neurosci. 2019 Jul 10;39(28):5481-5492. doi: 10.1523/JNEUROSCI.0227-18.2019. Epub 2019 May 28. J Neurosci. 2019. PMID: 31138656 Free PMC article.
-
Enhancement of ventricular-subventricular zone-derived neurogenesis and oligodendrogenesis by erythropoietin and its derivatives.Front Cell Neurosci. 2013 Nov 27;7:235. doi: 10.3389/fncel.2013.00235. Front Cell Neurosci. 2013. PMID: 24348331 Free PMC article. Review.
-
Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain.Cells. 2022 May 31;11(11):1809. doi: 10.3390/cells11111809. Cells. 2022. PMID: 35681504 Free PMC article. Review.
-
Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone.Glia. 2012 Oct;60(10):1451-67. doi: 10.1002/glia.22365. Epub 2012 Jun 17. Glia. 2012. PMID: 22706957 Free PMC article.
-
Cerebrospinal fluid derived from progressive multiple sclerosis patients promotes neuronal and oligodendroglial differentiation of human neural precursor cells in vitro.Neuroscience. 2013 Oct 10;250:614-21. doi: 10.1016/j.neuroscience.2013.07.022. Epub 2013 Jul 19. Neuroscience. 2013. PMID: 23876320
Cited by
-
The Subventricular Zone in Glioblastoma: Genesis, Maintenance, and Modeling.Front Oncol. 2022 Mar 10;12:790976. doi: 10.3389/fonc.2022.790976. eCollection 2022. Front Oncol. 2022. PMID: 35359410 Free PMC article. Review.
-
Functional Heterogeneity of Mouse and Human Brain OPCs: Relevance for Preclinical Studies in Multiple Sclerosis.J Clin Med. 2020 Jun 2;9(6):1681. doi: 10.3390/jcm9061681. J Clin Med. 2020. PMID: 32498223 Free PMC article.
-
A radical scavenger edaravone inhibits matrix metalloproteinase-9 upregulation and blood-brain barrier breakdown in a mouse model of prolonged cerebral hypoperfusion.Neurosci Lett. 2014 Jun 24;573:40-45. doi: 10.1016/j.neulet.2014.05.005. Epub 2014 May 10. Neurosci Lett. 2014. PMID: 24820542 Free PMC article.
-
In Vitro Effects of Methylprednisolone over Oligodendroglial Cells: Foresight to Future Cell Therapies.Cells. 2023 May 30;12(11):1515. doi: 10.3390/cells12111515. Cells. 2023. PMID: 37296635 Free PMC article.
-
Transplantation of iPS-derived vascular endothelial cells improves white matter ischemic damage.J Neurochem. 2020 Jun;153(6):759-771. doi: 10.1111/jnc.14949. Epub 2020 Jan 21. J Neurochem. 2020. PMID: 31883380 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

