STAT2 phosphorylation and signaling
- PMID: 24416652
- PMCID: PMC3876438
- DOI: 10.4161/jkst.25790
STAT2 phosphorylation and signaling
Abstract
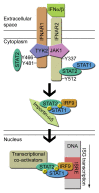
STAT2 is an essential transcription factor in type I IFN mediated anti-viral and anti-proliferative signaling. STAT2 function is regulated by tyrosine phosphorylation, which is the trigger for STAT-dimerization, subsequent nuclear translocation, and transcriptional activation of IFN stimulated genes. Evidence of additional STAT2 phosphorylation sites has emerged as well as novel roles for STAT2 separate from the classical ISGF3-signaling. This review aims to summarize knowledge of phosphorylation-mediated STAT2-regulation and future avenues of related STAT2 research.
Keywords: STAT2; interferon; phosphorylation; receptors; regulation.
Figures

Similar articles
-
The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review.
-
The proximal tyrosines of the cytoplasmic domain of the beta chain of the type I interferon receptor are essential for signal transducer and activator of transcription (Stat) 2 activation. Evidence that two Stat2 sites are required to reach a threshold of interferon alpha-induced Stat2 tyrosine phosphorylation that allows normal formation of interferon-stimulated gene factor 3.J Biol Chem. 1999 Feb 12;274(7):4045-52. doi: 10.1074/jbc.274.7.4045. J Biol Chem. 1999. PMID: 9933596
-
STAT2/IRF9 directs a prolonged ISGF3-like transcriptional response and antiviral activity in the absence of STAT1.Biochem J. 2015 Mar 15;466(3):511-24. doi: 10.1042/BJ20140644. Biochem J. 2015. PMID: 25564224 Free PMC article.
-
Distinct STAT structure promotes interaction of STAT2 with the p48 subunit of the interferon-alpha-stimulated transcription factor ISGF3.J Biol Chem. 1997 Aug 8;272(32):20070-6. doi: 10.1074/jbc.272.32.20070. J Biol Chem. 1997. PMID: 9242679
-
A Positive Feedback Amplifier Circuit That Regulates Interferon (IFN)-Stimulated Gene Expression and Controls Type I and Type II IFN Responses.Front Immunol. 2018 May 28;9:1135. doi: 10.3389/fimmu.2018.01135. eCollection 2018. Front Immunol. 2018. PMID: 29892288 Free PMC article. Review.
Cited by
-
Stat2 stability regulation: an intersection between immunity and carcinogenesis.Exp Mol Med. 2020 Sep;52(9):1526-1536. doi: 10.1038/s12276-020-00506-6. Epub 2020 Sep 25. Exp Mol Med. 2020. PMID: 32973222 Free PMC article. Review.
-
A Phenotypic Switch of Differentiated Glial Cells to Dedifferentiated Cells Is Regulated by Folate Receptor α.Stem Cells. 2019 Nov;37(11):1441-1454. doi: 10.1002/stem.3067. Epub 2019 Aug 14. Stem Cells. 2019. PMID: 31381815 Free PMC article.
-
Altered Transcriptional Regulation of Glycolysis in Circulating CD8+ T Cells of Rheumatoid Arthritis Patients.Genes (Basel). 2022 Jul 7;13(7):1216. doi: 10.3390/genes13071216. Genes (Basel). 2022. PMID: 35886000 Free PMC article.
-
Inhibition of the IFN-α JAK/STAT Pathway by MERS-CoV and SARS-CoV-1 Proteins in Human Epithelial Cells.Viruses. 2022 Mar 23;14(4):667. doi: 10.3390/v14040667. Viruses. 2022. PMID: 35458397 Free PMC article.
-
Proteomic profiling of oleamide-mediated polarization in a primary human monocyte-derived tumor-associated macrophages (TAMs) model: a functional analysis.PeerJ. 2024 Sep 18;12:e18090. doi: 10.7717/peerj.18090. eCollection 2024. PeerJ. 2024. PMID: 39308806 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
