Evidence synthesis for medical decision making and the appropriate use of quality scores
- PMID: 24415749
- PMCID: PMC4453425
- DOI: 10.3121/cmr.2013.1188
Evidence synthesis for medical decision making and the appropriate use of quality scores
Abstract
Meta-analyses today continue to be run using conventional random-effects models that ignore tangible information from studies such as the quality of the studies involved, despite the expectation that results of better quality studies reflect more valid results. Previous research has suggested that quality scores derived from such quality appraisals are unlikely to be useful in meta-analysis, because they would produce biased estimates of effects that are unlikely to be offset by a variance reduction within the studied models. However, previous discussions took place in the context of such scores viewed in terms of their ability to maximize their association with both the magnitude and direction of bias. In this review, another look is taken at this concept, this time asserting that probabilistic bias quantification is not possible or even required of quality scores when used in meta-analysis for redistribution of weights. The use of such a model is contrasted with the conventional random effects model of meta-analysis to demonstrate why the latter is inadequate in the face of a properly specified quality score weighting method.
Keywords: Bias; Medical decision making; Meta-analysis; Quality scores.
© 2014 Marshfield Clinic.
Figures
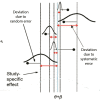
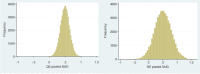

Comment in
-
Is it time for the Cochrane Collaboration to reconsider its meta-analysis methodology?Clin Med Res. 2014 Sep;12(1-2):2-3. doi: 10.3121/cmr.2013.1188-1209. Epub 2014 Feb 26. Clin Med Res. 2014. PMID: 24573703 Free PMC article. No abstract available.
Similar articles
-
Is it time for the Cochrane Collaboration to reconsider its meta-analysis methodology?Clin Med Res. 2014 Sep;12(1-2):2-3. doi: 10.3121/cmr.2013.1188-1209. Epub 2014 Feb 26. Clin Med Res. 2014. PMID: 24573703 Free PMC article. No abstract available.
-
On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions.Biostatistics. 2001 Dec;2(4):463-71. doi: 10.1093/biostatistics/2.4.463. Biostatistics. 2001. PMID: 12933636
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review.J Evid Based Med. 2015 Feb;8(1):2-10. doi: 10.1111/jebm.12141. J Evid Based Med. 2015. PMID: 25594108 Review.
-
Evidence-Based Decision-Making 2: Systematic Reviews and Meta-Analysis.Methods Mol Biol. 2021;2249:405-428. doi: 10.1007/978-1-0716-1138-8_22. Methods Mol Biol. 2021. PMID: 33871856 Review.
Cited by
-
Guidance on the use of the weight of evidence approach in scientific assessments.EFSA J. 2017 Aug 3;15(8):e04971. doi: 10.2903/j.efsa.2017.4971. eCollection 2017 Aug. EFSA J. 2017. PMID: 32625632 Free PMC article.
-
Is it time for the Cochrane Collaboration to reconsider its meta-analysis methodology?Clin Med Res. 2014 Sep;12(1-2):2-3. doi: 10.3121/cmr.2013.1188-1209. Epub 2014 Feb 26. Clin Med Res. 2014. PMID: 24573703 Free PMC article. No abstract available.
-
Occupational noise and ischemic heart disease: A systematic review.Noise Health. 2016 Jul-Aug;18(83):167-77. doi: 10.4103/1463-1741.189241. Noise Health. 2016. PMID: 27569404 Free PMC article. Review.
-
Long-term noise exposure and the risk for type 2 diabetes: a meta-analysis.Noise Health. 2015 Jan-Feb;17(74):23-33. doi: 10.4103/1463-1741.149571. Noise Health. 2015. PMID: 25599755 Free PMC article.
-
Application of the Ipswich Touch Test for diabetic peripheral neuropathy screening: a systematic review and meta-analysis.BMJ Open. 2021 Oct 4;11(10):e046966. doi: 10.1136/bmjopen-2020-046966. BMJ Open. 2021. PMID: 34607858 Free PMC article.
References
-
- Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992;45:255–265. - PubMed
-
- Lohr KN. Rating the strength of scientific evidence: relevance for quality improvement programs. Int J Qual Health Care 2004;16:9–18. - PubMed
-
- Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2001;2:463–471. - PubMed
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–613. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
