Bacteriophage Associated Silicon Particles: Design and Characterization of a Novel Theranostic Vector with Improved Payload Carrying Potential
- PMID: 24409342
- PMCID: PMC3881592
- DOI: 10.1039/C3TB20595A
Bacteriophage Associated Silicon Particles: Design and Characterization of a Novel Theranostic Vector with Improved Payload Carrying Potential
Abstract
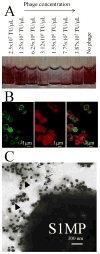
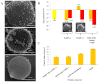
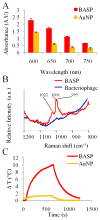
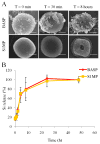
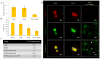
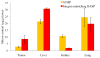
There has been extensive research on the use of nanovectors for cancer therapy. Targeted delivery of nanotherapeutics necessitates two important characteristics; the ability to accumulate at the disease locus after overcoming sequential biological barriers and the ability to carry a substantial therapeutic payload. Successful combination of the above two features is challenging, especially in solid porous materials where chemical conjugation of targeting entities on the particle surface will generally prevent successful loading of the therapeutic substance. In this study, we propose a novel strategy for decorating the surface of mesoporous silicon particles with targeting entities (bacteriophage) and gold nanoparticles (AuNP) while maintaining their payload carrying potential. The resulting Bacteriophage Associated Silicon Particles (BASP) demonstrates efficient encapsulation of macromolecules and therapeutic nanoparticles into the porous structures. In vitro targeting data show enhanced targeting efficiency with about four orders of magnitude lower concentration of bacteriophage. In vivo targeting data suggest that BASP maintain their integrity following intravenous administration in mice and display up to three fold higher accumulation in the tumor.
Figures



Similar articles
-
Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics.Acc Chem Res. 2011 Oct 18;44(10):979-89. doi: 10.1021/ar200077p. Epub 2011 Sep 8. Acc Chem Res. 2011. PMID: 21902173 Free PMC article. Review.
-
Preparation and in vivo evaluation of red blood cell membrane coated porous silicon nanoparticles implanted with 155Tb.Nucl Med Biol. 2020 May-Jun;84-85:102-110. doi: 10.1016/j.nucmedbio.2020.04.001. Epub 2020 Apr 10. Nucl Med Biol. 2020. PMID: 32334356
-
Identification of pancreatic tumors in vivo with ligand-targeted, pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography.J Control Release. 2016 Jun 10;231:60-7. doi: 10.1016/j.jconrel.2015.12.055. Epub 2016 Jan 5. J Control Release. 2016. PMID: 26763377 Free PMC article.
-
Hyaluronic acid-capped compact silica-supported mesoporous titania nanoparticles for ligand-directed delivery of doxorubicin.Acta Biomater. 2018 Oct 15;80:364-377. doi: 10.1016/j.actbio.2018.09.006. Epub 2018 Sep 8. Acta Biomater. 2018. PMID: 30201431
-
PRINT: a novel platform toward shape and size specific nanoparticle theranostics.Acc Chem Res. 2011 Oct 18;44(10):990-8. doi: 10.1021/ar2000315. Epub 2011 Aug 2. Acc Chem Res. 2011. PMID: 21809808 Free PMC article. Review.
Cited by
-
Redirecting Transport of Nanoparticle Albumin-Bound Paclitaxel to Macrophages Enhances Therapeutic Efficacy against Liver Metastases.Cancer Res. 2016 Jan 15;76(2):429-39. doi: 10.1158/0008-5472.CAN-15-1576. Epub 2016 Jan 7. Cancer Res. 2016. PMID: 26744528 Free PMC article.
-
Design and Preparation of "corn-like" SPIONs@DFK-SBP-M13 Assembly for Improvement of Effective Internalization.Int J Nanomedicine. 2021 Oct 19;16:7091-7102. doi: 10.2147/IJN.S325282. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34703229 Free PMC article.
-
Modeling of Nanotherapy Response as a Function of the Tumor Microenvironment: Focus on Liver Metastasis.Front Bioeng Biotechnol. 2020 Aug 19;8:1011. doi: 10.3389/fbioe.2020.01011. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974325 Free PMC article. Review.
-
Chemically Modifying Viruses for Diverse Applications.ACS Chem Biol. 2016 May 20;11(5):1167-79. doi: 10.1021/acschembio.6b00060. Epub 2016 Mar 21. ACS Chem Biol. 2016. PMID: 26930417 Free PMC article. Review.
-
Low pressure mediated enhancement of nanoparticle and macromolecule loading into porous silicon structures.Mesoporous Biomater. 2014;1(1):10.2478/mesbi-2014-0002. doi: 10.2478/mesbi-2014-0002. Mesoporous Biomater. 2014. PMID: 25485265 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

