A new animal model of spontaneous autoimmune peripheral polyneuropathy: implications for Guillain-Barré syndrome
- PMID: 24401681
- PMCID: PMC3895684
- DOI: 10.1186/2051-5960-2-5
A new animal model of spontaneous autoimmune peripheral polyneuropathy: implications for Guillain-Barré syndrome
Abstract
Background: Spontaneous autoimmune peripheral neuropathy including Guillain-Barré Syndrome (GBS) represents as one of the serious emergencies in neurology. Although pathological changes have been well documented, molecular and cellular mechanisms of GBS are still under-explored, partially due to short of appropriate animal models. The field lacks of spontaneous and translatable models for mechanistic investigations. As GBS is preceded often by viral or bacterial infection, a condition can enhance co-stimulatory activity; we sought to investigate the critical role of T cell co-stimulation in this autoimmune disease.
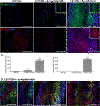
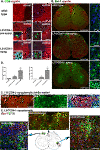
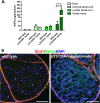
Results: Our previous study reported that transgene-derived constitutive expression of co-stimulator B7.2 on antigen presenting cells of the nervous tissues drove spontaneous neurological disorders. Depletion of CD4+ T cells in L31 mice accelerated the onset and increased the prevalence of the disease. In the current study, we further demonstrated that L31/CD4-/- mice exhibited both motor and sensory deficits, including weakness and paresis of limbs, numbness to mechanical stimuli and hypersensitivity to thermal stimulation. Pathological changes were characterized by massive infiltration of macrophages and CD8+ T cells, demyelination and axonal damage in peripheral nerves, while changes in spinal cords could be secondary to the PNS damage. In symptomatic L31/CD4-/- mice, the disruption of the blood neural barriers was observed mainly in peripheral nerves. Interestingly, the infiltration of immune cells was initiated in pre-symptomatic L31/CD4-/- mice, prior to the disease onset, in the DRG and spinal roots where the blood nerve barrier is virtually absent.
Conclusions: L31/CD4-/- mice mimic most parts of clinical and pathological signatures of GBS in human; thus providing an unconventional opportunity to experimentally explore the critical events that lead to spontaneous, autoimmune demyelinating disease of the peripheral nervous system.
Figures




Similar articles
-
CX3CR1 But Not CCR2 Expression Is Required for the Development of Autoimmune Peripheral Neuropathy in Mice.Front Immunol. 2021 Aug 16;12:720733. doi: 10.3389/fimmu.2021.720733. eCollection 2021. Front Immunol. 2021. PMID: 34484228 Free PMC article.
-
Evidence from Human and Animal Studies: Pathological Roles of CD8(+) T Cells in Autoimmune Peripheral Neuropathies.Front Immunol. 2015 Oct 15;6:532. doi: 10.3389/fimmu.2015.00532. eCollection 2015. Front Immunol. 2015. PMID: 26528293 Free PMC article. Review.
-
CD4 T cells mediate axonal damage and spinal cord motor neuron apoptosis in murine p0106-125-induced experimental autoimmune neuritis.Am J Pathol. 2008 Jul;173(1):93-105. doi: 10.2353/ajpath.2008.071101. Epub 2008 Jun 5. Am J Pathol. 2008. PMID: 18535178 Free PMC article.
-
Effector/memory CD8+ T cells synergize with co-stimulation competent macrophages to trigger autoimmune peripheral neuropathy.Brain Behav Immun. 2018 Jul;71:142-157. doi: 10.1016/j.bbi.2018.04.001. Epub 2018 Apr 5. Brain Behav Immun. 2018. PMID: 29627532
-
Axonal degeneration in Guillain-Barré syndrome: a reappraisal.J Neurol. 2021 Oct;268(10):3728-3743. doi: 10.1007/s00415-020-10034-y. Epub 2020 Jun 30. J Neurol. 2021. PMID: 32607643 Review.
Cited by
-
Impaired dendritic cell function in a spontaneous autoimmune polyneuropathy.J Immunol. 2015 May 1;194(9):4175-84. doi: 10.4049/jimmunol.1401766. Epub 2015 Mar 30. J Immunol. 2015. PMID: 25825437 Free PMC article.
-
The Pathogenesis of the Demyelinating Form of Guillain-Barre Syndrome (GBS): Proteo-peptidomic and Immunological Profiling of Physiological Fluids.Mol Cell Proteomics. 2016 Jul;15(7):2366-78. doi: 10.1074/mcp.M115.056036. Epub 2016 May 3. Mol Cell Proteomics. 2016. PMID: 27143409 Free PMC article.
-
Hyperbaric oxygen therapy improves recovery at acute motor axonal neuropathy case.J Neurosci Rural Pract. 2023 Jan-Mar;14(1):145-148. doi: 10.25259/JNRP_9_2022. Epub 2022 Dec 21. J Neurosci Rural Pract. 2023. PMID: 36891088 Free PMC article.
-
Barrier function in the peripheral and central nervous system-a review.Pflugers Arch. 2017 Jan;469(1):123-134. doi: 10.1007/s00424-016-1920-8. Epub 2016 Dec 12. Pflugers Arch. 2017. PMID: 27957611 Review.
-
Zika virus infection causes temporary paralysis in adult mice with motor neuron synaptic retraction and evidence for proximal peripheral neuropathy.Sci Rep. 2019 Dec 20;9(1):19531. doi: 10.1038/s41598-019-55717-3. Sci Rep. 2019. PMID: 31862897 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

