The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25
- PMID: 24399297
- PMCID: PMC4008495
- DOI: 10.1126/scisignal.2004577
The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25
Abstract
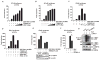
Ubiquitylation is an important mechanism for regulating innate immune responses to viral infections. Attachment of lysine 63 (Lys(63))-linked ubiquitin chains to the RNA sensor retinoic acid-inducible gene-I (RIG-I) by the ubiquitin E3 ligase tripartite motif protein 25 (TRIM25) leads to the activation of RIG-I and stimulates production of the antiviral cytokines interferon-α (IFN-α) and IFN-β. Conversely, Lys(48)-linked ubiquitylation of TRIM25 by the linear ubiquitin assembly complex (LUBAC) stimulates the proteasomal degradation of TRIM25, thereby inhibiting the RIG-I signaling pathway. Here, we report that ubiquitin-specific protease 15 (USP15) deubiquitylates TRIM25, preventing the LUBAC-dependent degradation of TRIM25. Through protein purification and mass spectrometry analysis, we identified USP15 as an interaction partner of TRIM25 in human cells. Knockdown of endogenous USP15 by specific small interfering RNA markedly enhanced the ubiquitylation of TRIM25. In contrast, expression of wild-type USP15, but not its catalytically inactive mutant, reduced the Lys(48)-linked ubiquitylation of TRIM25, leading to its stabilization. Furthermore, ectopic expression of USP15 enhanced the TRIM25- and RIG-I-dependent production of type I IFN and suppressed RNA virus replication. In contrast, depletion of USP15 resulted in decreased IFN production and markedly enhanced viral replication. Together, these data identify USP15 as a critical regulator of the TRIM25- and RIG-I-mediated antiviral immune response, thereby highlighting the intricate regulation of innate immune signaling.
Conflict of interest statement
Figures




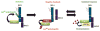
Similar articles
-
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling.J Virol. 2018 Feb 26;92(6):e01737-17. doi: 10.1128/JVI.01737-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263274 Free PMC article.
-
Activation of duck RIG-I by TRIM25 is independent of anchored ubiquitin.PLoS One. 2014 Jan 23;9(1):e86968. doi: 10.1371/journal.pone.0086968. eCollection 2014. PLoS One. 2014. PMID: 24466302 Free PMC article.
-
Negative regulation of RIG-I-mediated antiviral signaling by TRK-fused gene (TFG) protein.Biochem Biophys Res Commun. 2013 Jul 19;437(1):168-72. doi: 10.1016/j.bbrc.2013.06.061. Epub 2013 Jun 26. Biochem Biophys Res Commun. 2013. PMID: 23810392
-
Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review.
-
TRIM proteins: another class of viral victims.Sci Signal. 2010 Apr 20;3(118):jc2. doi: 10.1126/scisignal.3118jc2. Sci Signal. 2010. PMID: 20407122 Review.
Cited by
-
USP15 Deubiquitinates TUT1 Associated with RNA Metabolism and Maintains Cerebellar Homeostasis.Mol Cell Biol. 2020 Oct 13;40(21):e00098-20. doi: 10.1128/MCB.00098-20. Print 2020 Oct 13. Mol Cell Biol. 2020. PMID: 32839293 Free PMC article.
-
Ubiquitin-specific Protease 15 Negatively Regulates Virus-induced Type I Interferon Signaling via Catalytically-dependent and -independent Mechanisms.Sci Rep. 2015 Jun 10;5:11220. doi: 10.1038/srep11220. Sci Rep. 2015. PMID: 26061460 Free PMC article.
-
USP4 function and multifaceted roles in cancer: a possible and potential therapeutic target.Cancer Cell Int. 2020 Jul 10;20:298. doi: 10.1186/s12935-020-01391-9. eCollection 2020. Cancer Cell Int. 2020. PMID: 32669974 Free PMC article. Review.
-
USP15 regulates type I interferon response and is required for pathogenesis of neuroinflammation.Nat Immunol. 2017 Jan;18(1):54-63. doi: 10.1038/ni.3581. Epub 2016 Oct 10. Nat Immunol. 2017. PMID: 27721430
-
Zebrafish TRIM25 Promotes Innate Immune Response to RGNNV Infection by Targeting 2CARD and RD Regions of RIG-I for K63-Linked Ubiquitination.Front Immunol. 2019 Dec 3;10:2805. doi: 10.3389/fimmu.2019.02805. eCollection 2019. Front Immunol. 2019. PMID: 31849979 Free PMC article.
References
-
- Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. - PubMed
-
- Nakhaei P, Genin P, Civas A, Hiscott J. RIG-I-like receptors: Sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. - PubMed
-
- Hornung V, Ellegast J, Kim S, Brzoóka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. - PubMed
-
- Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. - PubMed
-
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

