A novel keratin18 promoter that drives reporter gene expression in the intrahepatic and extrahepatic biliary system allows isolation of cell-type specific transcripts from zebrafish liver
- PMID: 24394404
- PMCID: PMC4677818
- DOI: 10.1016/j.gep.2013.12.002
A novel keratin18 promoter that drives reporter gene expression in the intrahepatic and extrahepatic biliary system allows isolation of cell-type specific transcripts from zebrafish liver
Abstract
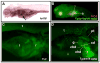
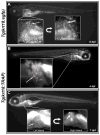
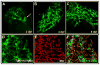
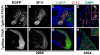
Heritable and acquired biliary disorders are an important cause of acute and chronic human liver disease. Biliary development and physiology have been studied extensively in rodent models and more recently, zebrafish have been used to uncover pathogenic mechanisms and potential therapies for these conditions. Here we report development of novel transgenic lines labeling the intrahepatic and extrahepatic biliary system of zebrafish larvae that can be used for lineage tracing and isolation of biliary-specific RNAs from mixed populations of liver cells. We show that GFP expression driven by a 4.4 kilobase promoter fragment from the zebrafish keratin18 (krt18) gene allows visualization of all components of the developing biliary system as early as 3 days post-fertilization. In addition, expression of a ribosomal fusion protein (EGFP-Rpl10a) in krt18:TRAP transgenic fish allows for enrichment of translated biliary cell mRNAs via translating ribosome affinity purification (TRAP). Future studies utilizing these reagents will enhance our understanding of the morphologic and molecular processes involved in biliary development and disease.
Keywords: Gallbladder; Gene profiling; Liver; Translation.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
An Apo-14 promoter-driven transgenic zebrafish that marks liver organogenesis.PLoS One. 2011;6(7):e22555. doi: 10.1371/journal.pone.0022555. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799896 Free PMC article.
-
The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish.BMC Dev Biol. 2007 May 4;7:42. doi: 10.1186/1471-213X-7-42. BMC Dev Biol. 2007. PMID: 17477879 Free PMC article.
-
Development of translating ribosome affinity purification for zebrafish.Genesis. 2013 Mar;51(3):187-92. doi: 10.1002/dvg.22363. Epub 2013 Feb 26. Genesis. 2013. PMID: 23281262 Free PMC article.
-
Development of the Intrahepatic and Extrahepatic Biliary Tract: A Framework for Understanding Congenital Diseases.Annu Rev Pathol. 2020 Jan 24;15:1-22. doi: 10.1146/annurev-pathmechdis-012418-013013. Epub 2019 Jul 12. Annu Rev Pathol. 2020. PMID: 31299162 Review.
-
Generation and application of signaling pathway reporter lines in zebrafish.Mol Genet Genomics. 2013 Jun;288(5-6):231-42. doi: 10.1007/s00438-013-0750-z. Epub 2013 May 15. Mol Genet Genomics. 2013. PMID: 23674148 Free PMC article. Review.
Cited by
-
Chronically high level of tgfb1a induction causes both hepatocellular carcinoma and cholangiocarcinoma via a dominant Erk pathway in zebrafish.Oncotarget. 2017 Aug 18;8(44):77096-77109. doi: 10.18632/oncotarget.20357. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100373 Free PMC article.
-
Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing.Int J Mol Sci. 2021 Dec 14;22(24):13417. doi: 10.3390/ijms222413417. Int J Mol Sci. 2021. PMID: 34948215 Free PMC article. Review.
-
Zebrafish: an important tool for liver disease research.Gastroenterology. 2015 Nov;149(6):1361-77. doi: 10.1053/j.gastro.2015.08.034. Epub 2015 Aug 28. Gastroenterology. 2015. PMID: 26319012 Free PMC article. Review.
-
Core Hippo pathway components act as a brake on Yap and Taz in the development and maintenance of the biliary network.Development. 2020 Jun 22;147(12):dev184242. doi: 10.1242/dev.184242. Development. 2020. PMID: 32439761 Free PMC article.
-
Impaired Redox and Protein Homeostasis as Risk Factors and Therapeutic Targets in Toxin-Induced Biliary Atresia.Gastroenterology. 2020 Sep;159(3):1068-1084.e2. doi: 10.1053/j.gastro.2020.05.080. Epub 2020 Jun 4. Gastroenterology. 2020. PMID: 32505743 Free PMC article.
References
-
- Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. - PubMed
-
- Kawakami K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 2004;77:201–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

