STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes
- PMID: 24391507
- PMCID: PMC3879373
- DOI: 10.1371/journal.ppat.1003861
STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes
Abstract
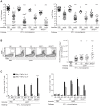
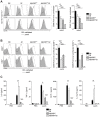
Infection with Listeria monocytogenes strains that enter the host cell cytosol leads to a robust cytotoxic T cell response resulting in long-lived cell-mediated immunity (CMI). Upon entry into the cytosol, L. monocytogenes secretes cyclic diadenosine monophosphate (c-di-AMP) which activates the innate immune sensor STING leading to the expression of IFN-β and co-regulated genes. In this study, we examined the role of STING in the development of protective CMI to L. monocytogenes. Mice deficient for STING or its downstream effector IRF3 restricted a secondary lethal challenge with L. monocytogenes and exhibited enhanced immunity that was MyD88-independent. Conversely, enhancing STING activation during immunization by co-administration of c-di-AMP or by infection with a L. monocytogenes mutant that secretes elevated levels of c-di-AMP resulted in decreased protective immunity that was largely dependent on the type I interferon receptor. These data suggest that L. monocytogenes activation of STING downregulates CMI by induction of type I interferon.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: Daniel A. Portnoy has a consulting relationship with a financial interest in Aduro Biotech, and both he and the company stand to benefit from the commercialization of this research. This does not alter our adherence to all PLOS polices on sharing data and materials.
Figures






Similar articles
-
Secretion of c-di-AMP by Listeria monocytogenes Leads to a STING-Dependent Antibacterial Response during Enterocolitis.Infect Immun. 2020 Nov 16;88(12):e00407-20. doi: 10.1128/IAI.00407-20. Print 2020 Nov 16. Infect Immun. 2020. PMID: 33020211 Free PMC article.
-
The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides.Infect Immun. 2011 Feb;79(2):688-94. doi: 10.1128/IAI.00999-10. Epub 2010 Nov 22. Infect Immun. 2011. PMID: 21098106 Free PMC article.
-
Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity.Adv Immunol. 2012;113:135-56. doi: 10.1016/B978-0-12-394590-7.00002-6. Adv Immunol. 2012. PMID: 22244582 Review.
-
The Complement Anaphylatoxins C5a and C3a Suppress IFN-β Production in Response to Listeria monocytogenes by Inhibition of the Cyclic Dinucleotide-Activated Cytosolic Surveillance Pathway.J Immunol. 2017 Apr 15;198(8):3237-3244. doi: 10.4049/jimmunol.1601420. Epub 2017 Mar 8. J Immunol. 2017. PMID: 28275134 Free PMC article.
-
Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells?Cell Microbiol. 2020 Apr;22(4):e13175. doi: 10.1111/cmi.13175. Cell Microbiol. 2020. PMID: 32185899 Free PMC article. Review.
Cited by
-
The human P-glycoprotein transporter enhances the type I interferon response to Listeria monocytogenes infection.Infect Immun. 2015 Jun;83(6):2358-68. doi: 10.1128/IAI.00380-15. Epub 2015 Mar 30. Infect Immun. 2015. PMID: 25824830 Free PMC article.
-
STING controls T cell memory fitness during infection through T cell-intrinsic and IDO-dependent mechanisms.Proc Natl Acad Sci U S A. 2023 Jan 17;120(3):e2205049120. doi: 10.1073/pnas.2205049120. Epub 2023 Jan 12. Proc Natl Acad Sci U S A. 2023. PMID: 36634134 Free PMC article.
-
Cyclooxygenase-1 and -2 Play Contrasting Roles in Listeria-Stimulated Immunity.J Immunol. 2018 Jun 1;200(11):3729-3738. doi: 10.4049/jimmunol.1700701. Epub 2018 Apr 20. J Immunol. 2018. PMID: 29678951 Free PMC article.
-
Unraveling the convoluted biological roles of type I interferons in infection and immunity: a way forward for therapeutics and vaccine design.Front Immunol. 2014 Aug 29;5:412. doi: 10.3389/fimmu.2014.00412. eCollection 2014. Front Immunol. 2014. PMID: 25221557 Free PMC article. Review.
-
In Vivo Synthesis of Cyclic-di-GMP Using a Recombinant Adenovirus Preferentially Improves Adaptive Immune Responses against Extracellular Antigens.J Immunol. 2016 Feb 15;196(4):1741-52. doi: 10.4049/jimmunol.1501272. Epub 2016 Jan 20. J Immunol. 2016. PMID: 26792800 Free PMC article.
References
-
- Harty JT, Tvinnereim AR, White DW (2000) CD8+ T cell effector mechanisms in resistance to infection. Annual review of immunology 18: 275–308. - PubMed
-
- Pamer EG (2004) Immune responses to Listeria monocytogenes. Nat Rev Immunol 4: 812–823. - PubMed
-
- Schenten D, Medzhitov R (2011) The control of adaptive immune responses by the innate immune system. Advances in immunology 109: 87–124. - PubMed
-
- Witte CE, Archer KA, Rae CS, Sauer JD, Woodward JJ, et al. (2012) Innate immune pathways triggered by Listeria monocytogenes and their role in the induction of cell-mediated immunity. Advances in immunology 113: 135–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

