The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells
- PMID: 24391212
- PMCID: PMC3905741
- DOI: 10.4049/jimmunol.1300720
The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells
Abstract
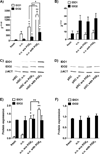
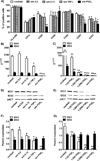
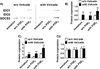
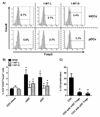
Dendritic cells (DCs) are professional APCs that have a role in the initiation of adaptive immune responses and tolerance. Among the tolerogenic mechanisms, the expression of the enzyme IDO1 represents an effective tool to generate T regulatory cells. In humans, different DC subsets express IDO1, but less is known about the IDO1-related enzyme IDO2. In this study, we found a different pattern of expression and regulation between IDO1 and IDO2 in human circulating DCs. At the protein level, IDO1 is expressed only in circulating myeloid DCs (mDCs) and is modulated by PGE2, whereas IDO2 is expressed in both mDCs and plasmacytoid DCs and is not modulated by PGE2. In healthy subjects, IDO1 expression requires the presence of PGE2 and needs continuous transcription and translation, whereas IDO2 expression is constitutive, independent from suppressor of cytokine signaling 3 activity. Conversely, in patients suffering from inflammatory arthritis, circulating DCs express both IDO1 and IDO2. At the functional level, both mDCs and plasmacytoid DCs generate T regulatory cells through an IDO1/IDO2-dependent mechanism. We conclude that, in humans, whereas IDO1 provides an additional mechanism of tolerance induced by proinflammatory mediators, IDO2 is stably expressed in steady-state conditions and may contribute to the homeostatic tolerogenic capacity of DCs.
Conflict of interest statement
Figures
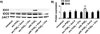
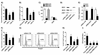
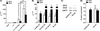
Similar articles
-
PGE2-induced IDO1 inhibits the capacity of fully mature DCs to elicit an in vitro antileukemic immune response.J Immunol Res. 2015;2015:253191. doi: 10.1155/2015/253191. Epub 2015 Feb 26. J Immunol Res. 2015. PMID: 25815345 Free PMC article.
-
LPS enhances CTB-INSULIN induction of IDO1 and IL-10 synthesis in human dendritic cells.Cell Immunol. 2019 Apr;338:32-42. doi: 10.1016/j.cellimm.2019.03.003. Epub 2019 Mar 19. Cell Immunol. 2019. PMID: 30910218 Free PMC article.
-
IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation.Int Immunol. 2014 Jul;26(7):357-67. doi: 10.1093/intimm/dxt073. Epub 2014 Jan 8. Int Immunol. 2014. PMID: 24402311 Free PMC article.
-
T cell regulatory plasmacytoid dendritic cells expressing indoleamine 2,3 dioxygenase.Handb Exp Pharmacol. 2009;(188):165-96. doi: 10.1007/978-3-540-71029-5_8. Handb Exp Pharmacol. 2009. PMID: 19031026 Free PMC article. Review.
-
Impact of IDO1 and IDO2 on the B Cell Immune Response.Front Immunol. 2022 Apr 13;13:886225. doi: 10.3389/fimmu.2022.886225. eCollection 2022. Front Immunol. 2022. PMID: 35493480 Free PMC article. Review.
Cited by
-
Host IDO2 Gene Status Influences Tumor Progression and Radiotherapy Response in KRAS-Driven Sporadic Pancreatic Cancers.Clin Cancer Res. 2019 Jan 15;25(2):724-734. doi: 10.1158/1078-0432.CCR-18-0814. Epub 2018 Sep 28. Clin Cancer Res. 2019. PMID: 30266763 Free PMC article.
-
Immune cell engineering: opportunities in lung cancer therapeutics.Drug Deliv Transl Res. 2020 Oct;10(5):1203-1227. doi: 10.1007/s13346-020-00719-2. Drug Deliv Transl Res. 2020. PMID: 32172351 Review.
-
Human type 1 and type 2 conventional dendritic cells express indoleamine 2,3-dioxygenase 1 with functional effects on T cell priming.Eur J Immunol. 2021 Jun;51(6):1494-1504. doi: 10.1002/eji.202048580. Epub 2021 Mar 22. Eur J Immunol. 2021. PMID: 33675038 Free PMC article.
-
Tryptophan-degrading enzymes in tumoral immune resistance.Front Immunol. 2015 Feb 3;6:34. doi: 10.3389/fimmu.2015.00034. eCollection 2015. Front Immunol. 2015. PMID: 25691885 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase 2 immunohistochemical expression in medullary thyroid carcinoma: implications in prognosis and immunomodulatory effects.BMC Cancer. 2022 Nov 1;22(1):1116. doi: 10.1186/s12885-022-10173-7. BMC Cancer. 2022. PMID: 36319978 Free PMC article.
References
-
- MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–1520. - PubMed
-
- Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J. Exp. Med. 1996;184:695–706. - PMC - PubMed
-
- Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J. Immunol. 1996;157:1406–1414. - PubMed
-
- Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today. 1999;20:469–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

