pH sensing and regulation in cancer
- PMID: 24381558
- PMCID: PMC3865727
- DOI: 10.3389/fphys.2013.00370
pH sensing and regulation in cancer
Abstract
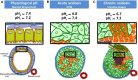
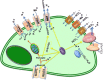
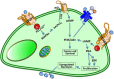
Cells maintain intracellular pH (pHi) within a narrow range (7.1-7.2) by controlling membrane proton pumps and transporters whose activity is set by intra-cytoplasmic pH sensors. These sensors have the ability to recognize and induce cellular responses to maintain the pHi, often at the expense of acidifying the extracellular pH. In turn, extracellular acidification impacts cells via specific acid-sensing ion channels (ASICs) and proton-sensing G-protein coupled receptors (GPCRs). In this review, we will discuss some of the major players in proton sensing at the plasma membrane and their downstream consequences in cancer cells and how these pH-mediated changes affect processes such as migration and metastasis. The complex mechanisms by which they transduce acid pH signals to the cytoplasm and nucleus are not well understood. However, there is evidence that expression of proton-sensing GPCRs such as GPR4, TDAG8, and OGR1 can regulate aspects of tumorigenesis and invasion, including cofilin and talin regulated actin (de-)polymerization. Major mechanisms for maintenance of pHi homeostasis include monocarboxylate, bicarbonate, and proton transporters. Notably, there is little evidence suggesting a link between their activities and those of the extracellular H(+)-sensors, suggesting a mechanistic disconnect between intra- and extracellular pH. Understanding the mechanisms of pH sensing and regulation may lead to novel and informed therapeutic strategies that can target acidosis, a common physical hallmark of solid tumors.
Keywords: buffer therapy; cancer microenvironment; extracellular acidification; intracellular pH; pH regulators; proton sensors.
Figures
Similar articles
-
pH sensing in skin tumors: Methods to study the involvement of GPCRs, acid-sensing ion channels and transient receptor potential vanilloid channels.Exp Dermatol. 2020 Nov;29(11):1055-1061. doi: 10.1111/exd.14150. Epub 2020 Aug 18. Exp Dermatol. 2020. PMID: 32658355
-
Extracellular acidification synergizes with PDGF to stimulate migration of mouse embryo fibroblasts through activation of p38MAPK with a PTX-sensitive manner.Biochem Biophys Res Commun. 2015 May 1;460(2):191-7. doi: 10.1016/j.bbrc.2015.03.006. Epub 2015 Mar 10. Biochem Biophys Res Commun. 2015. PMID: 25769958
-
Acidic tumor microenvironment and pH-sensing G protein-coupled receptors.Front Physiol. 2013 Dec 5;4:354. doi: 10.3389/fphys.2013.00354. Front Physiol. 2013. PMID: 24367336 Free PMC article. Review.
-
Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing.Exp Dermatol. 2017 Feb;26(2):127-132. doi: 10.1111/exd.13209. Exp Dermatol. 2017. PMID: 27623507 Review.
-
Regulation of inflammation by extracellular acidification and proton-sensing GPCRs.Cell Signal. 2013 Nov;25(11):2263-71. doi: 10.1016/j.cellsig.2013.07.022. Epub 2013 Jul 31. Cell Signal. 2013. PMID: 23917207 Review.
Cited by
-
Ca2+ -dependent interactions between lipids and the tumor-targeting peptide pHLIP.Protein Sci. 2022 Sep;31(9):e4385. doi: 10.1002/pro.4385. Protein Sci. 2022. PMID: 36040255 Free PMC article.
-
pH-sensing G protein-coupled orphan receptor GPR68 is expressed in human cartilage and correlates with degradation of extracellular matrix during OA progression.PeerJ. 2023 Dec 5;11:e16553. doi: 10.7717/peerj.16553. eCollection 2023. PeerJ. 2023. PMID: 38077417 Free PMC article.
-
T-cells produce acidic niches in lymph nodes to suppress their own effector functions.Nat Commun. 2020 Aug 17;11(1):4113. doi: 10.1038/s41467-020-17756-7. Nat Commun. 2020. PMID: 32807791 Free PMC article.
-
Does it make sense to target one tumor cell chemotactic factor or its receptor when several chemotactic axes are involved in metastasis of the same cancer?Clin Transl Med. 2016 Dec;5(1):28. doi: 10.1186/s40169-016-0113-6. Epub 2016 Aug 10. Clin Transl Med. 2016. PMID: 27510263 Free PMC article. Review.
-
Dynamic scenario of metabolic pathway adaptation in tumors and therapeutic approach.Oncoscience. 2015 Feb 9;2(3):225-32. doi: 10.18632/oncoscience.123. eCollection 2015. Oncoscience. 2015. PMID: 25897425 Free PMC article.
References
-
- Barathova M., Takacova M., Holotnakova T., Gibadulinova A., Ohradanova A., Zatovicova M., et al. (2008). Alternative splicing variant of the hypoxia marker carbonic anhydrase IX expressed independently of hypoxia and tumor phenotype. Br. J. Cancer 98, 129–136 10.1038/sj.bjc.6604111 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

