Gene dosage-dependent rescue of HSP neurite defects in SPG4 patients' neurons
- PMID: 24381312
- PMCID: PMC3990156
- DOI: 10.1093/hmg/ddt644
Gene dosage-dependent rescue of HSP neurite defects in SPG4 patients' neurons
Abstract
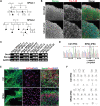
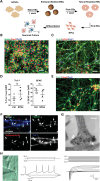
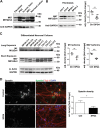
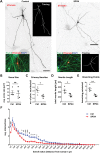
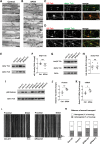
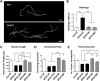
The hereditary spastic paraplegias (HSPs) are a heterogeneous group of motorneuron diseases characterized by progressive spasticity and paresis of the lower limbs. Mutations in Spastic Gait 4 (SPG4), encoding spastin, are the most frequent cause of HSP. To understand how mutations in SPG4 affect human neurons, we generated human induced pluripotent stem cells (hiPSCs) from fibroblasts of two patients carrying a c.1684C>T nonsense mutation and from two controls. These SPG4 and control hiPSCs were able to differentiate into neurons and glia at comparable efficiency. All known spastin isoforms were reduced in SPG4 neuronal cells. The complexity of SPG4 neurites was decreased, which was paralleled by an imbalance of axonal transport with less retrograde movement. Prominent neurite swellings with disrupted microtubules were present in SPG4 neurons at an ultrastructural level. While some of these swellings contain acetylated and detyrosinated tubulin, these tubulin modifications were unchanged in total cell lysates of SPG4 neurons. Upregulation of another microtubule-severing protein, p60 katanin, may partially compensate for microtubuli dynamics in SPG4 neurons. Overexpression of the M1 or M87 spastin isoforms restored neurite length, branching, numbers of primary neurites and reduced swellings in SPG4 neuronal cells. We conclude that neurite complexity and maintenance in HSP patient-derived neurons are critically sensitive to spastin gene dosage. Our data show that elevation of single spastin isoform levels is sufficient to restore neurite complexity and reduce neurite swellings in patient cells. Furthermore, our human model offers an ideal platform for pharmacological screenings with the goal to restore physiological spastin levels in SPG4 patients.
Figures
Similar articles
-
Loss of spastin function results in disease-specific axonal defects in human pluripotent stem cell-based models of hereditary spastic paraplegia.Stem Cells. 2014 Feb;32(2):414-23. doi: 10.1002/stem.1569. Stem Cells. 2014. PMID: 24123785 Free PMC article.
-
Pleiotropic effects of spastin on neurite growth depending on expression levels.J Neurochem. 2009 Mar;108(5):1277-88. doi: 10.1111/j.1471-4159.2009.05875.x. Epub 2009 Jan 29. J Neurochem. 2009. PMID: 19141076
-
Hereditary spastic paraplegia SPG4: what is known and not known about the disease.Brain. 2015 Sep;138(Pt 9):2471-84. doi: 10.1093/brain/awv178. Epub 2015 Jun 20. Brain. 2015. PMID: 26094131 Free PMC article. Review.
-
Evaluation of loss of function as an explanation for SPG4-based hereditary spastic paraplegia.Hum Mol Genet. 2010 Jul 15;19(14):2767-79. doi: 10.1093/hmg/ddq177. Epub 2010 Apr 29. Hum Mol Genet. 2010. PMID: 20430936 Free PMC article.
-
Autosomal dominant spastic paraplegias: a review of 89 families resulting from a portuguese survey.JAMA Neurol. 2013 Apr;70(4):481-7. doi: 10.1001/jamaneurol.2013.1956. JAMA Neurol. 2013. PMID: 23400676 Review.
Cited by
-
Utility of Induced Pluripotent Stem Cells for the Study and Treatment of Genetic Diseases: Focus on Childhood Neurological Disorders.Front Mol Neurosci. 2016 Sep 6;9:78. doi: 10.3389/fnmol.2016.00078. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27656126 Free PMC article. Review.
-
Hereditary Spastic Paraplegia: An Update.Int J Mol Sci. 2022 Feb 1;23(3):1697. doi: 10.3390/ijms23031697. Int J Mol Sci. 2022. PMID: 35163618 Free PMC article. Review.
-
The Quest for Anti-α-Synuclein Antibody Specificity-Lessons Learnt From Flow Cytometry Analysis.Front Neurol. 2022 Jul 15;13:869103. doi: 10.3389/fneur.2022.869103. eCollection 2022. Front Neurol. 2022. PMID: 35911883 Free PMC article.
-
How neurons maintain their axons long-term: an integrated view of axon biology and pathology.Front Neurosci. 2023 Jul 26;17:1236815. doi: 10.3389/fnins.2023.1236815. eCollection 2023. Front Neurosci. 2023. PMID: 37564364 Free PMC article. Review.
-
Aberrant NOVA1 function disrupts alternative splicing in early stages of amyotrophic lateral sclerosis.Acta Neuropathol. 2022 Sep;144(3):413-435. doi: 10.1007/s00401-022-02450-3. Epub 2022 Jul 1. Acta Neuropathol. 2022. PMID: 35778567 Free PMC article.
References
-
- Kasher P.R., De Vos K.J., Wharton S.B., Manser C., Bennett E.J., Bingley M., Wood J.D., Milner R., McDermott C.J., Miller C.C., et al. Direct evidence for axonal transport defects in a novel mouse model of mutant spastin-induced hereditary spastic paraplegia (HSP) and human HSP patients. J. Neurochem. 2009;110:34–44. - PubMed
-
- McDermott C.J., Grierson A.J., Wood J.D., Bingley M., Wharton S.B., Bushby K.M.D., Shaw P.J. Hereditary spastic paraparesis: disrupted intracellular transport associated with spastin mutation. Ann. Neurol. 2003;54:748–759. - PubMed
-
- Wharton S.B., McDermott C.J., Grierson A.J., Wood J.D., Gelsthorpe C., Ince P.G., Shaw P.J. The cellular and molecular pathology of the motor system in hereditary spastic paraparesis due to mutation of the spastin gene. J. Neuropathol. Exp. Neurol. 2003;62:1166–1177. - PubMed
-
- Hazan J., Fonknechten N., Mavel D., Paternotte C., Samson D., Artiguenave F., Davoine C.S., Cruaud C., Durr A., Wincker P., et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat. Genet. 1999;23:296–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

