Functional validation of a human CAPN5 exome variant by lentiviral transduction into mouse retina
- PMID: 24381307
- PMCID: PMC3990166
- DOI: 10.1093/hmg/ddt661
Functional validation of a human CAPN5 exome variant by lentiviral transduction into mouse retina
Abstract
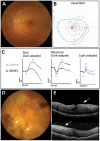
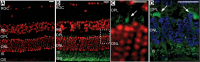
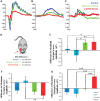
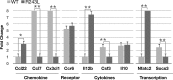
Exome sequencing indicated that the gene encoding the calpain-5 protease, CAPN5, is the likely cause of retinal degeneration and autoimmune uveitis in human patients with autosomal dominant neovascular inflammatory vitreoretinopathy (ADNIV, OMIM #193235). To explore the mechanism of ADNIV, a human CAPN5 disease allele was expressed in mouse retinas with a lentiviral vector created to express either the wild-type human (h) CAPN5 or the ADNIV mutant hCAPN5-R243L allele under a rhodopsin promoter with tandem green fluorescent protein (GFP) expression. Vectors were injected into the subretinal space of perinatal mice. Mouse phenotypes were analyzed using electroretinography, histology and inflammatory gene expression profiling. Mouse calpain-5 showed high homology to its human ortholog with >98% sequence identity that includes the ADNIV mutant residue. Calpain-5 protein was expressed in the inner and outer segments of the photoreceptors and in the outer plexiform layer. Expression of the hCAPN5-R243L allele caused loss of the electroretinogram b-wave, photoreceptor degeneration and induction of immune cell infiltration and inflammatory genes in the retina, recapitulating major features of the ADNIV phenotype. Intraocular neovascularization and fibrosis were not observed during the study period. Our study shows that expression of the hCAPN5-R243L disease allele elicits an ADNIV-like disease in mice. It further suggests that ADNIV is due to CAPN5 gain-of-function rather than haploinsufficiency, and retinal expression may be sufficient to generate an autoimmune response. Genetic models of ADNIV in the mouse can be used to explore protease mechanisms in retinal degeneration and inflammation as well as preclinical therapeutic testing.
Figures




Similar articles
-
CAPN5 mutation in hereditary uveitis: the R243L mutation increases calpain catalytic activity and triggers intraocular inflammation in a mouse model.Hum Mol Genet. 2015 Aug 15;24(16):4584-98. doi: 10.1093/hmg/ddv189. Epub 2015 May 20. Hum Mol Genet. 2015. PMID: 25994508 Free PMC article.
-
Photoreceptor Cell-Derived CAPN5 Regulates Retinal Pigment Epithelium Cell Proliferation Through Direct Regulation of SLIT2 Cleavage.Invest Ophthalmol Vis Sci. 2018 Apr 1;59(5):1810-1821. doi: 10.1167/iovs.17-22689. Invest Ophthalmol Vis Sci. 2018. PMID: 29610848
-
Structural modeling of a novel CAPN5 mutation that causes uveitis and neovascular retinal detachment.PLoS One. 2015 Apr 9;10(4):e0122352. doi: 10.1371/journal.pone.0122352. eCollection 2015. PLoS One. 2015. PMID: 25856303 Free PMC article.
-
Autosomal dominant neovascular inflammatory vitreoretinopathy with CAPN5 c.731T > C gene mutation; clinical management of a family cohort and review of the literature.Ophthalmic Genet. 2023 Dec;44(6):559-567. doi: 10.1080/13816810.2023.2255257. Epub 2023 Nov 20. Ophthalmic Genet. 2023. PMID: 37782277 Review.
-
Retinal degeneration mutants in the mouse.Vision Res. 2002 Feb;42(4):517-25. doi: 10.1016/s0042-6989(01)00146-8. Vision Res. 2002. PMID: 11853768 Review.
Cited by
-
Two Novel CAPN5 Variants Associated with Mild and Severe Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy Phenotypes.Ocul Immunol Inflamm. 2019;27(5):693-698. doi: 10.1080/09273948.2017.1370651. Epub 2017 Oct 17. Ocul Immunol Inflamm. 2019. PMID: 29040051 Free PMC article.
-
Calpain-5 Expression in the Retina Localizes to Photoreceptor Synapses.Invest Ophthalmol Vis Sci. 2016 May 1;57(6):2509-21. doi: 10.1167/iovs.15-18680. Invest Ophthalmol Vis Sci. 2016. PMID: 27152965 Free PMC article.
-
Molecular Surgery: Proteomics of a Rare Genetic Disease Gives Insight into Common Causes of Blindness.iScience. 2020 Oct 13;23(11):101667. doi: 10.1016/j.isci.2020.101667. eCollection 2020 Nov 20. iScience. 2020. PMID: 33134897 Free PMC article. Review.
-
Long-term progression of retinal degeneration in a preclinical model of CLN7 Batten disease as a baseline for testing clinical therapeutics.EBioMedicine. 2022 Nov;85:104314. doi: 10.1016/j.ebiom.2022.104314. Epub 2022 Oct 29. EBioMedicine. 2022. PMID: 36374771 Free PMC article.
-
Capn5 Expression in the Healthy and Regenerating Zebrafish Retina.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3643-3654. doi: 10.1167/iovs.18-24278. Invest Ophthalmol Vis Sci. 2018. PMID: 30029251 Free PMC article.
References
-
- Pastor J.C., de la Rua E.R., Martin F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog. Retin. Eye Res. 2002;21:127–144. - PubMed
-
- Frank R.N. Diabetic retinopathy. N. Engl. J. Med. 2004;350:48–58. - PubMed
-
- Syntichaki P., Xu K., Driscoll M., Tavernarakis N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature. 2002;419:939–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01EY018213/EY/NEI NIH HHS/United States
- 5T32EY013933/EY/NEI NIH HHS/United States
- R01 EY018213/EY/NEI NIH HHS/United States
- F32 EY022280/EY/NEI NIH HHS/United States
- K08 EY020530/EY/NEI NIH HHS/United States
- 5P30CA013696/CA/NCI NIH HHS/United States
- 5T32DK007647-20/DK/NIDDK NIH HHS/United States
- T32 EY013933/EY/NEI NIH HHS/United States
- 5P30EY019007/EY/NEI NIH HHS/United States
- R01 EY024665/EY/NEI NIH HHS/United States
- 08EY020530/EY/NEI NIH HHS/United States
- 1F32EY022280-01A1/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

