Proteotoxicity: an underappreciated pathology in cardiac disease
- PMID: 24380730
- PMCID: PMC4011959
- DOI: 10.1016/j.yjmcc.2013.12.015
Proteotoxicity: an underappreciated pathology in cardiac disease
Abstract
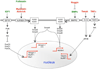
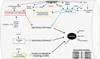
In general, in most organ systems, intracellular protein homeostasis is the sum of many factors, including chromosomal state, protein synthesis, post-translational processing and transport, folding, assembly and disassembly into macromolecular complexes, protein stability and clearance. In the heart, there has been a focus on the gene programs that are activated during pathogenic processes, but the removal of damaged proteins and organelles has been underappreciated as playing an important role in the pathogenesis of heart disease. Proteotoxicity refers to the adverse effects of damaged or misfolded proteins and even organelles on the cell. At the cellular level, the ultimate outcome of uncontrolled or severe proteotoxicity is cell death; hence, the pathogenic impact of proteotoxicity is maximally manifested in organs with no or very poor regenerative capability such as the brain and the heart. Evidence for increased cardiac proteotoxicity is rapidly mounting for a large subset of congenital and acquired human heart disease. Studies carried out in animal models and in cell culture have begun to establish both sufficiency and, in some cases, the necessity of proteotoxicity as a major pathogenic factor in the heart. This dictates rigorous testing for the efficacy of proteotoxic attenuation as a new strategy to treat heart disease. This review article highlights some recent advances in our understanding of how misfolded proteins can injure and are handled in the cell, examining the emerging evidence for targeting proteotoxicity as a new therapeutic strategy for heart disease. This article is part of a Special Issue entitled "Protein Quality Control, the Ubiquitin Proteasome System, and Autophagy."
Keywords: Autophagy; Lysosome; Protein.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Proteasomal and lysosomal protein degradation and heart disease.J Mol Cell Cardiol. 2014 Jun;71:16-24. doi: 10.1016/j.yjmcc.2013.11.006. Epub 2013 Nov 14. J Mol Cell Cardiol. 2014. PMID: 24239609 Free PMC article. Review.
-
The interplay between autophagy and the ubiquitin-proteasome system in cardiac proteotoxicity.Biochim Biophys Acta. 2015 Feb;1852(2):188-94. doi: 10.1016/j.bbadis.2014.07.028. Epub 2014 Aug 1. Biochim Biophys Acta. 2015. PMID: 25092168 Free PMC article. Review.
-
Priming the Proteasome to Protect against Proteotoxicity.Trends Mol Med. 2020 Jul;26(7):639-648. doi: 10.1016/j.molmed.2020.02.007. Epub 2020 Mar 26. Trends Mol Med. 2020. PMID: 32589934 Free PMC article. Review.
-
p62 Stages an interplay between the ubiquitin-proteasome system and autophagy in the heart of defense against proteotoxic stress.Trends Cardiovasc Med. 2011 Nov;21(8):224-8. doi: 10.1016/j.tcm.2012.05.015. Trends Cardiovasc Med. 2011. PMID: 22902070 Free PMC article. Review.
-
The heart of autophagy: deconstructing cardiac proteotoxicity.Autophagy. 2008 Oct;4(7):932-5. doi: 10.4161/auto.6756. Epub 2008 Oct 8. Autophagy. 2008. PMID: 18769158 Free PMC article.
Cited by
-
Sensing, signaling and surviving mitochondrial stress.Cell Mol Life Sci. 2021 Aug;78(16):5925-5951. doi: 10.1007/s00018-021-03887-7. Epub 2021 Jul 6. Cell Mol Life Sci. 2021. PMID: 34228161 Free PMC article. Review.
-
Glutathione: A Samsonian life-sustaining small molecule that protects against oxidative stress, ageing and damaging inflammation.Front Nutr. 2022 Nov 1;9:1007816. doi: 10.3389/fnut.2022.1007816. eCollection 2022. Front Nutr. 2022. PMID: 36386929 Free PMC article. Review.
-
NEDD8 Ultimate Buster 1 Long (NUB1L) Protein Suppresses Atypical Neddylation and Promotes the Proteasomal Degradation of Misfolded Proteins.J Biol Chem. 2015 Sep 25;290(39):23850-62. doi: 10.1074/jbc.M115.664375. Epub 2015 Aug 10. J Biol Chem. 2015. PMID: 26260793 Free PMC article.
-
Ube2v1 Positively Regulates Protein Aggregation by Modulating Ubiquitin Proteasome System Performance Partially Through K63 Ubiquitination.Circ Res. 2020 Mar 27;126(7):907-922. doi: 10.1161/CIRCRESAHA.119.316444. Epub 2020 Feb 21. Circ Res. 2020. PMID: 32081062 Free PMC article.
-
Effect of Tim23 knockdown in vivo on mitochondrial protein import and retrograde signaling to the UPRmt in muscle.Am J Physiol Cell Physiol. 2018 Oct 1;315(4):C516-C526. doi: 10.1152/ajpcell.00275.2017. Epub 2018 Jun 27. Am J Physiol Cell Physiol. 2018. PMID: 29949403 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

