Astrocytic and vascular remodeling in the injured adult rat spinal cord after chondroitinase ABC treatment
- PMID: 24380419
- PMCID: PMC3996996
- DOI: 10.1089/neu.2013.3143
Astrocytic and vascular remodeling in the injured adult rat spinal cord after chondroitinase ABC treatment
Abstract
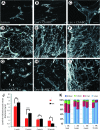
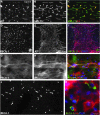
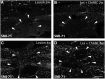
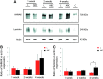
Upregulation of extracellular chondroitin sulfate proteoglycans (CSPG) is a primary cause for the failure of axons to regenerate after spinal cord injury (SCI), and the beneficial effect of their degradation by chondroitinase ABC (ChABC) is widely documented. Little is known, however, about the effect of ChABC treatment on astrogliosis and revascularization, two important factors influencing axon regrowth. This was investigated in the present study. Immediately after a spinal cord hemisection at thoracic level 8-9, we injected ChABC intrathecally at the sacral level, repeated three times until 10 days post-injury. Our results show an effective cleavage of CSPG glycosaminoglycan chains and stimulation of axonal remodeling within the injury site, accompanied by an extended period of astrocyte remodeling (up to 4 weeks). Interestingly, ChABC treatment favored an orientation of astrocytic processes directed toward the injury, in close association with axons at the lesion entry zone, suggesting a correlation between axon and astrocyte remodeling. Further, during the first weeks post-injury, ChABC treatment affected the morphology of laminin-positive blood vessel basement membranes and vessel-independent laminin deposits: hypertrophied blood vessels with detached or duplicated basement membrane were more numerous than in lesioned untreated animals. In contrast, at later time points, laminin expression increased and became more directly associated with newly formed blood vessels, the size of which tended to be closer to that found in intact tissue. Our data reinforce the idea that ChABC injection in combination with other synergistic treatments is a promising therapeutic strategy for SCI repair.
Figures



Similar articles
-
IT delivery of ChABC modulates NG2 and promotes GAP-43 axonal regrowth after spinal cord injury.Cell Mol Neurobiol. 2011 Nov;31(8):1129-39. doi: 10.1007/s10571-011-9714-1. Epub 2011 Jun 1. Cell Mol Neurobiol. 2011. PMID: 21630006
-
Benefit of chondroitinase ABC on sensory axon regeneration in a laceration model of spinal cord injury in the rat.Surg Neurol. 2008 Jun;69(6):568-77; discussion 577. doi: 10.1016/j.surneu.2008.02.009. Surg Neurol. 2008. PMID: 18486695 Free PMC article.
-
Axonal regeneration of Clarke's neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC.Exp Neurol. 2003 Jul;182(1):160-8. doi: 10.1016/s0014-4886(02)00052-3. Exp Neurol. 2003. PMID: 12821386
-
Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury.Brain Res Bull. 2011 Mar 10;84(4-5):306-16. doi: 10.1016/j.brainresbull.2010.06.015. Epub 2010 Jul 8. Brain Res Bull. 2011. PMID: 20620201 Review.
-
Injured mice at the gym: review, results and considerations for combining chondroitinase and locomotor exercise to enhance recovery after spinal cord injury.Brain Res Bull. 2011 Mar 10;84(4-5):317-26. doi: 10.1016/j.brainresbull.2010.06.002. Epub 2010 Jun 15. Brain Res Bull. 2011. PMID: 20558254 Free PMC article. Review.
Cited by
-
Astrocytes Transplanted during Early Postnatal Development Integrate, Mature, and Survive Long Term in Mouse Cortex.J Neurosci. 2023 Mar 1;43(9):1509-1529. doi: 10.1523/JNEUROSCI.0544-22.2023. Epub 2023 Jan 20. J Neurosci. 2023. PMID: 36669885 Free PMC article.
-
Beneficial Effects of Resveratrol-Mediated Inhibition of the mTOR Pathway in Spinal Cord Injury.Neural Plast. 2018 Mar 26;2018:7513748. doi: 10.1155/2018/7513748. eCollection 2018. Neural Plast. 2018. PMID: 29780409 Free PMC article. Review.
-
Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury.Sci Rep. 2017 Aug 21;7(1):9018. doi: 10.1038/s41598-017-09432-6. Sci Rep. 2017. PMID: 28827771 Free PMC article.
-
Revascularization After Traumatic Spinal Cord Injury.Front Physiol. 2021 Apr 30;12:631500. doi: 10.3389/fphys.2021.631500. eCollection 2021. Front Physiol. 2021. PMID: 33995118 Free PMC article. Review.
-
Peripheral Nerve Transplantation Combined with Acidic Fibroblast Growth Factor and Chondroitinase Induces Regeneration and Improves Urinary Function in Complete Spinal Cord Transected Adult Mice.PLoS One. 2015 Oct 1;10(10):e0139335. doi: 10.1371/journal.pone.0139335. eCollection 2015. PLoS One. 2015. PMID: 26426529 Free PMC article.
References
-
- Fitch M.T., and Silver J. (1997). Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol. 148, 587–603 - PubMed
-
- Rhodes K.E., Raivich G., and Fawcett J.W. (2006). The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience 140, 87–100 - PubMed
-
- Silver J., and Miller J.H. (2004). Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 - PubMed
-
- Sofroniew M. V. (2000). Astrocyte failure as a cause of CNS dysfunction. Mol. Psychiatry 5, 230–232 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

