Cytoplasmic protein methylation is essential for neural crest migration
- PMID: 24379414
- PMCID: PMC3882789
- DOI: 10.1083/jcb.201306071
Cytoplasmic protein methylation is essential for neural crest migration
Abstract
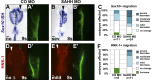
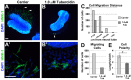
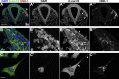
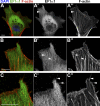
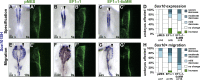
As they initiate migration in vertebrate embryos, neural crest cells are enriched for methylation cycle enzymes, including S-adenosylhomocysteine hydrolase (SAHH), the only known enzyme to hydrolyze the feedback inhibitor of trans-methylation reactions. The importance of methylation in neural crest migration is unknown. Here, we show that SAHH is required for emigration of polarized neural crest cells, indicating that methylation is essential for neural crest migration. Although nuclear histone methylation regulates neural crest gene expression, SAHH and lysine-methylated proteins are abundant in the cytoplasm of migratory neural crest cells. Proteomic profiling of cytoplasmic, lysine-methylated proteins from migratory neural crest cells identified 182 proteins, several of which are cytoskeleton related. A methylation-resistant form of one of these proteins, the actin-binding protein elongation factor 1 alpha 1 (EF1α1), blocks neural crest migration. Altogether, these data reveal a novel and essential role for post-translational nonhistone protein methylation during neural crest migration and define a previously unknown requirement for EF1α1 methylation in migration.
Figures



Similar articles
-
Expression of actin-binding proteins and requirement for actin-depolymerizing factor in chick neural crest cells.Dev Dyn. 2014 May;243(5):730-8. doi: 10.1002/dvdy.24105. Dev Dyn. 2014. PMID: 24868596 Free PMC article.
-
Novel insight into the function and regulation of alphaN-catenin by Snail2 during chick neural crest cell migration.Dev Biol. 2010 Aug 15;344(2):896-910. doi: 10.1016/j.ydbio.2010.06.006. Epub 2010 Jun 11. Dev Biol. 2010. PMID: 20542025 Free PMC article.
-
Cellular localization and signaling activity of beta-catenin in migrating neural crest cells.Dev Dyn. 2004 Aug;230(4):708-26. doi: 10.1002/dvdy.20091. Dev Dyn. 2004. PMID: 15254905
-
Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis.Dev Dyn. 2004 Jan;229(1):14-29. doi: 10.1002/dvdy.10485. Dev Dyn. 2004. PMID: 14699574 Review.
-
The "beginnings" of the neural crest.Dev Biol. 2018 Dec 1;444 Suppl 1:S3-S13. doi: 10.1016/j.ydbio.2018.07.019. Epub 2018 Jul 23. Dev Biol. 2018. PMID: 30048640 Review.
Cited by
-
On the Need to Tell Apart Fraternal Twins eEF1A1 and eEF1A2, and Their Respective Outfits.Int J Mol Sci. 2021 Jun 28;22(13):6973. doi: 10.3390/ijms22136973. Int J Mol Sci. 2021. PMID: 34203525 Free PMC article. Review.
-
Neural crest migration: trailblazing ahead.F1000Prime Rep. 2015 Jan 5;7:02. doi: 10.12703/P7-02. eCollection 2015. F1000Prime Rep. 2015. PMID: 25705385 Free PMC article. Review.
-
Identification and Characterization of Two New S-Adenosylmethionine-Dependent Methyltransferase Encoding Genes Suggested Their Involvement in Stipe Elongation of Flammulina velutipes.Mycobiology. 2019 Sep 13;47(4):441-448. doi: 10.1080/12298093.2019.1658332. eCollection 2019. Mycobiology. 2019. PMID: 32010465 Free PMC article.
-
One crisis, diverse impacts-Tissue-specificity of folate deficiency-induced circulation defects in zebrafish larvae.PLoS One. 2017 Nov 27;12(11):e0188585. doi: 10.1371/journal.pone.0188585. eCollection 2017. PLoS One. 2017. PMID: 29176804 Free PMC article.
-
Expression of actin-binding proteins and requirement for actin-depolymerizing factor in chick neural crest cells.Dev Dyn. 2014 May;243(5):730-8. doi: 10.1002/dvdy.24105. Dev Dyn. 2014. PMID: 24868596 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

